Magnetic properties of a material are a class of physical phenomena mediated by fields. Electric currents and magnetic moments of elementary particles generate a field that acts on other currents. The most familiar effects occur in ferromagnetic materials, which are strongly attracted by magnetic fields and can become permanently magnetized, creating charged fields themselves.
Only a few substances are ferromagnetic. To determine the level of development of this phenomenon in a particular substance, there is a classification of materials according to magnetic properties. The most common are iron, nickel and cobalt and their alloys. The prefix ferro- refers to iron because permanent magnetism was first observed in an empty form of natural iron ore called magnetic properties of the material, Fe3O4.
You may be interested in: Technology “Pedagogical Workshop”: concept, main functions, characteristics of implementation and analysis of effectiveness
Paramagnetic materials
Although ferromagnetism is responsible for most of the effects of magnetism found in everyday life, all other materials are affected to some extent by the field, as well as some other types of magnetism. Paramagnetic substances such as aluminum and oxygen are weakly attracted to an applied magnetic field. Diamagnetic substances such as copper and carbon repel weakly.
You might be interested in: What are the endings for letters in English?
While antiferromagnetic materials such as chromium and spin glasses have a more complex relationship with the magnetic field. The magnetic strength on paramagnetic, diamagnetic, and antiferromagnetic materials is usually too weak to be felt and can only be detected by laboratory instruments, so these substances are not included in the list of materials that have magnetic properties.
Ferrites
Ferrites are a class of magnetic materials consisting of oxides of iron (Fe2O3) and other metals (NiO, MgO, ZnO, MnO, CuO, BaO, etc.). The composition of ferrites can be written by the formula
MeO⋅Fe2o3, where
Me is a divalent metal.
The components included in ferrites form between themselves vast areas of solid solutions, in which magnetic materials with a very wide range of properties are present. These materials can be magnetically hard or magnetically soft.
Ferrite production process
The ferrite production process is a complex set of technological operations, since the electromagnetic properties of ferrites change with minor deviations from the composition of the charge, the granularity of the powders, the specific pressure during pressing, the temperature and sintering time.
The ferrite production process consists of the following steps:
- preparation, mixing, grinding and annealing of the charge;
- introduction of plasticizers, second mixing with grinding and rubbing of the charge;
- pressing and sintering.
Depending on the composition of the ferrites, they are sintered at temperatures from 900 to 1400 °C in air. However, in some cases an inert environment is used. After firing, the products are checked for absence of cracks, chips, retention of configuration and dimensions, as well as for electromagnetic parameters.
The magnetic properties of ferrites depend on the chemical composition, sintering conditions and subsequent cooling conditions. Depending on these conditions, ferrites can have an initial magnetic permeability from unity to 4000. The saturation induction of ferrites is not high. So, at fields of 8–12 kA/m, the saturation induction is no more than 0.4 T. Ferrites are difficult to magnetize, and complete magnetic saturation occurs in very strong fields.
The electrical resistivity of ferrites varies within 0.1·105 Ohm·m, while for metals it is no more than 10-6 mOhm⋅. Ferrites are compounds of complex structural structure. The most common are spinel-type ferrites, whose unit cells are similar to the natural mineral MgO⋅Al2O3. There are ferrites with a hexagonal lattice, the structure of which is similar to the natural material Pb(Fe·Mn)12O19. In addition, there are ferrites with a unit cell similar to the natural mineral garnet and perovskite-type ferrites, similar in structure to the natural mineral CaO⋅TiO2.
Ferrites are used for the manufacture of parts for radios, televisions, storage and computing devices, magnetic recording systems, and as a structural material for building communication elements.
New promising magnetic materials are permanent magnets based on rare earth metals and amorphous magnetic materials. Magnets based on rare earth metals are compounds of rare earth elements with cobalt of the type:
RCO5, where
– R–Sm, Pr, Cd, Ce.
They have high magnetic energy (250 – 290 mJ/m3) and are used in microwave devices, aviation, space and other industries.
Amorphous magnetic materials have a composition that can be described by the formula:
T75-83M25-17, where
- T – Fe, Co, Ni (there may be microadditives of other metals);
- M – P, C, B, Si, Al.
Amorphous materials do not have grain boundaries, and the magnitude of the coercive force in them is vanishingly small (about 0.5 A/m). They are used to make magnetic shields, magnetic recording heads, relay cores and other products.
Story
The material's magnetic properties were first discovered in the ancient world when people noticed that magnets, naturally magnetized pieces of minerals, could attract iron. The word "magnet" comes from the Greek term μαγνῆτις λίθος magnētis lithos, "magnesium stone, footstone."
You may be interested in: Ulyanovsk State Agricultural Academy named after Stolypin
In ancient Greece, Aristotle credited the first of what can be called scientific discussions about the magnetic properties of materials to the philosopher Thales of Miletus, who lived from 625 BC. e. to 545 BC e. The ancient Indian medical text Sushruta Samhita describes the use of magnetite to remove arrows embedded in the human body.
Ancient China
In ancient China, the earliest literary reference to the electrical and magnetic properties of materials is in a 4th century BC book named after its author, The Sage of the Valley of Ghosts. The earliest mention of the attraction of a needle is in the 1st century work Lunheng (Balanced Queries): “The magnet attracts the needle.”
The 11th-century Chinese scientist Shen Kuo was the first person to describe—in the Dream Pool Essay—the magnetic needle compass and how it improved the accuracy of navigation through astronomical methods. True north concept. By the 12th century, the Chinese were known to use a magnetic compass for navigation. They fashioned a guide spoon out of stone so that the handle of the spoon always pointed south.
Magnetic hardness and softness
The phenomenon of hysteresis greatly affects the magnetic properties of materials. Substances in which the loop on the hysteresis graph is widened, requiring a significant coercive force for demagnetization, are called hard magnetic; materials with a narrow loop, which are much easier to demagnetize, are called soft magnetic.
In alternating fields, magnetic hysteresis manifests itself especially clearly. It is always accompanied by the release of heat. In addition, in an alternating magnetic field, eddy induction currents arise in the magnet, which generate especially a lot of heat.
Many ferromagnets and ferrimagnets are used in equipment operating on alternating current (for example, electromagnet cores) and are constantly remagnetized during operation. In order to reduce energy losses due to hysteresis and dynamic losses due to eddy currents, soft magnetic materials such as pure iron, ferrites, electrical steels, and alloys (for example, permalloy) are used in such equipment. There are other ways to minimize energy loss.
Hard magnetic substances, on the contrary, are used in equipment operating in a constant magnetic field. They retain residual magnetization much longer, but are more difficult to magnetize to saturation. Many of them are now composites of various types, such as cermet or neodymium magnets.
Middle Ages
Alexander Neckam, by 1187, was the first in Europe to describe the compass and its use for navigation. This researcher was the first in Europe to thoroughly establish what properties magnetic materials have. In 1269, Peter Peregrine de Maricourt wrote the Epistola de magnete, the first surviving treatise describing the properties of magnets. In 1282, the properties of compasses and materials with special magnetic properties were described by al-Ashraf, a Yemeni physicist, astronomer and geographer.
Literature
- Aksenovich L. A. Physics in secondary school: Theory. Tasks. Tests: Textbook. allowance for institutions providing general education. environment, education / L. A. Aksenovich, N. N. Rakina, K. S. Farino; Ed. K. S. Farino. - Mn.: Adukatsiya i vyakhavanne, 2004. - P.330-335.
- Zhilko, V.V. Physics: textbook. allowance for 11th grade. general education school from Russian language training / V.V. Zhilko, A.V. Lavrinenko, L. G. Markovich. — Mn.: Nar. Asveta, 2002. - pp. 291-297.
- Slobodyanyuk A.I. Physics 10. §13 Interaction of a magnetic field with matter
Renaissance
In 1600, William Gilbert published his Magnetic Corpus and Magnetic Tellurium (On the Magnet and Magnetic Bodies, and the Great Magnet of the Earth). In this work he describes many of his experiments with his model earth, called a terrella, with which he conducted research into the properties of magnetic materials.
From his experiments, he came to the conclusion that the Earth itself was magnetic and that this was why compasses pointed north (previously, some believed that it was the North Star (Polaris) or the large magnetic island at the North Pole that attracted the compass).
Magnetic poles and magnetic field.
The magnetic properties of a bar magnet are most noticeable near its ends. If such a magnet is hung by the middle part so that it can rotate freely in a horizontal plane, then it will take a position approximately corresponding to the direction from north to south. The end of the rod pointing north is called the north pole, and the opposite end is called the south pole. Opposite poles of two magnets attract each other, and like poles repel each other.
Also on topic:
EARTH'S MAGNETIC FIELD
If a bar of non-magnetized iron is brought close to one of the poles of a magnet, the latter will become temporarily magnetized. In this case, the pole of the magnetized bar closest to the pole of the magnet will be opposite in name, and the far one will have the same name. The attraction between the pole of the magnet and the opposite pole induced by it in the bar explains the action of the magnet. Some materials (such as steel) themselves become weak permanent magnets after being near a permanent magnet or electromagnet. A steel rod can be magnetized by simply passing the end of a bar permanent magnet along its end.
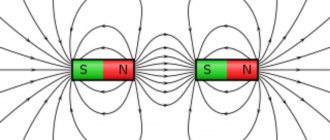
So, a magnet attracts other magnets and objects made of magnetic materials without being in contact with them. This action at a distance is explained by the existence of a magnetic field in the space around the magnet. Some idea of the intensity and direction of this magnetic field can be obtained by pouring iron filings onto a sheet of cardboard or glass placed on a magnet. The sawdust will line up in chains in the direction of the field, and the density of the sawdust lines will correspond to the intensity of this field. (They are thickest at the ends of the magnet, where the intensity of the magnetic field is greatest.)
M. Faraday (1791–1867) introduced the concept of closed induction lines for magnets. The induction lines extend into the surrounding space from the magnet at its north pole, enter the magnet at its south pole, and pass inside the magnet material from the south pole back to the north, forming a closed loop. The total number of induction lines emerging from a magnet is called magnetic flux. Magnetic flux density, or magnetic induction ( V
), is equal to the number of induction lines passing along the normal through an elementary area of unit size.
Magnetic induction determines the force with which a magnetic field acts on a current-carrying conductor located in it. If the conductor carrying current I
, is located perpendicular to the induction lines, then according to Ampere’s law the force
F
acting on the conductor is perpendicular to both the field and the conductor and is proportional to the magnetic induction, current strength and length of the conductor.
Thus, for magnetic induction B
we can write the expression
where F
– force in newtons,
I
– current in amperes,
l
– length in meters. The unit of measurement for magnetic induction is tesla (T). See also ELECTRICITY AND MAGNETISM.
New time
Understanding of the relationship between electricity and materials with special magnetic properties began in 1819 with the work of Hans Christian Ørsted, a professor at the University of Copenhagen, who discovered by accidentally twitching a compass needle near a wire that an electric current could create a magnetic field. This landmark experiment is known as the Oersted Experiment. Several other experiments followed with André-Marie Amperat, who in 1820 discovered that a magnetic field circulating along a closed path was coupled to a current flowing around the perimeter of the path.
You may be interested in: Regular hexagonal pyramid. Formulas for volume and surface area. Solution of a geometric problem
Carl Friedrich Gauss studied magnetism. Jean-Baptiste Biot and Felix Savart came up with the Biot-Savart law in 1820, which gives the desired equation. Michael Faraday, who in 1831 discovered that time-varying magnetic flux through a loop of wire caused a voltage. And other scientists found further connections between magnetism and electricity.
Magnetizing force and magnetic field strength.
Next, we should introduce another quantity characterizing the magnetic effect of electric current. Suppose that current passes through the wire of a long coil, inside of which there is a magnetizable material. The magnetizing force is the product of the electric current in the coil and the number of its turns (this force is measured in amperes, since the number of turns is a dimensionless quantity). Magnetic field strength H
equal to the magnetizing force per unit length of the coil.
Thus, the value of H
is measured in amperes per meter; it determines the magnetization acquired by the material inside the coil.
In a vacuum, magnetic induction B
proportional to the magnetic field strength
H
:
where m
0 – so-called
magnetic constant having a universal value of 4 p
× 10–7 H/m.
In many materials, the value of
B
is
approximately proportional
to H. However, in ferromagnetic materials the relationship between B
and
H
is somewhat more complex (as will be discussed below).
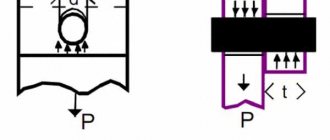
In Fig. 1 shows a simple electromagnet designed to grip loads. The energy source is a DC battery. The figure also shows the field lines of the electromagnet, which can be detected by the usual method of iron filings.
Large electromagnets with iron cores and a very large number of ampere-turns, operating in continuous mode, have a large magnetizing force. They create a magnetic induction of up to 6 Tesla in the gap between the poles; this induction is limited only by mechanical stress, heating of the coils and magnetic saturation of the core. A number of giant water-cooled electromagnets (without a core), as well as installations for creating pulsed magnetic fields, were designed by P.L. Kapitsa (1894–1984) in Cambridge and at the Institute of Physical Problems of the USSR Academy of Sciences and F. Bitter (1902–1967) in Massachusetts Institute of Technology. With such magnets it was possible to achieve induction of up to 50 Tesla. A relatively small electromagnet that produces fields of up to 6.2 Tesla, consumes 15 kW of electrical power and is cooled by liquid hydrogen, was developed at the Losalamos National Laboratory. Similar fields are obtained at cryogenic temperatures.
20th century and our time
James Clerk Maxwell synthesized and expanded this understanding of Maxwell's equations, combining electricity, magnetism, and optics into the field of electromagnetism. In 1905, Einstein used these laws to motivate his theory of special relativity, requiring that the laws hold true in all inertial frames of reference.
Electromagnetism continued to develop in the 21st century, being incorporated into more fundamental theories of gauge theory, quantum electrodynamics, electroweak theory, and finally the standard model. Nowadays, scientists are already studying the magnetic properties of nanostructured materials with all their might. But the greatest and most amazing discoveries in this field are probably still ahead of us.
Theories of magnetism.
For the first time, the guess that magnetic phenomena are ultimately reduced to electrical phenomena arose from Ampere in 1825, when he expressed the idea of closed internal microcurrents circulating in each atom of a magnet. However, without any experimental confirmation of the presence of such currents in matter (the electron was discovered by J. Thomson only in 1897, and the description of the structure of the atom was given by Rutherford and Bohr in 1913), this theory “faded.” In 1852, W. Weber suggested that each atom of a magnetic substance is a tiny magnet, or magnetic dipole, so that complete magnetization of a substance is achieved when all individual atomic magnets are aligned in a certain order (Fig. 4, b
). Weber believed that molecular or atomic “friction” helps these elementary magnets maintain their order despite the disturbing influence of thermal vibrations. His theory was able to explain the magnetization of bodies upon contact with a magnet, as well as their demagnetization upon impact or heating; finally, the “reproduction” of magnets when cutting a magnetized needle or magnetic rod into pieces was also explained. And yet this theory did not explain either the origin of the elementary magnets themselves, or the phenomena of saturation and hysteresis. Weber's theory was improved in 1890 by J. Ewing, who replaced his hypothesis of atomic friction with the idea of interatomic confining forces that help maintain the ordering of the elementary dipoles that make up a permanent magnet.
The approach to the problem, once proposed by Ampere, received a second life in 1905, when P. Langevin explained the behavior of paramagnetic materials by attributing to each atom an internal uncompensated electron current. According to Langevin, it is these currents that form tiny magnets that are randomly oriented when there is no external field, but acquire an orderly orientation when it is applied. In this case, the approach to complete order corresponds to saturation of magnetization. In addition, Langevin introduced the concept of a magnetic moment, which for an individual atomic magnet is equal to the product of the “magnetic charge” of a pole and the distance between the poles. Thus, the weak magnetism of paramagnetic materials is due to the total magnetic moment created by uncompensated electron currents.
In 1907, P. Weiss introduced the concept of “domain,” which became an important contribution to the modern theory of magnetism. Weiss imagined domains as small “colonies” of atoms, within which the magnetic moments of all atoms, for some reason, are forced to maintain the same orientation, so that each domain is magnetized to saturation. An individual domain can have linear dimensions of the order of 0.01 mm and, accordingly, a volume of the order of 10–6 mm3. The domains are separated by so-called Bloch walls, the thickness of which does not exceed 1000 atomic sizes. The “wall” and two oppositely oriented domains are shown schematically in Fig. 5. Such walls represent “transition layers” in which the direction of domain magnetization changes.
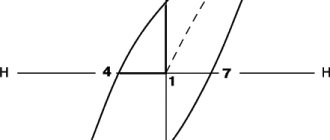
In the general case, three sections can be distinguished on the initial magnetization curve (Fig. 6). In the initial section, the wall, under the influence of an external field, moves through the thickness of the substance until it encounters a defect in the crystal lattice, which stops it. By increasing the field strength, you can force the wall to move further, through the middle section between the dashed lines. If after this the field strength is again reduced to zero, then the walls will no longer return to their original position, so the sample will remain partially magnetized. This explains the hysteresis of the magnet. At the final section of the curve, the process ends with the saturation of the magnetization of the sample due to the ordering of the magnetization inside the last disordered domains. This process is almost completely reversible. Magnetic hardness is exhibited by those materials whose atomic lattice contains many defects that impede the movement of interdomain walls. This can be achieved by mechanical and thermal treatment, for example by compression and subsequent sintering of the powdered material. In alnico alloys and their analogues, the same result is achieved by fusing metals into a complex structure.
In addition to paramagnetic and ferromagnetic materials, there are materials with so-called antiferromagnetic and ferrimagnetic properties. The difference between these types of magnetism is explained in Fig. 7. Based on the concept of domains, paramagnetism can be considered as a phenomenon caused by the presence in the material of small groups of magnetic dipoles, in which individual dipoles interact very weakly with each other (or do not interact at all) and therefore, in the absence of an external field, take only random orientations ( Fig. 7, a
).
In ferromagnetic materials, within each domain there is a strong interaction between individual dipoles, leading to their ordered parallel alignment (Fig. 7, b
).
In antiferromagnetic materials, on the contrary, the interaction between individual dipoles leads to their antiparallel ordered alignment, so that the total magnetic moment of each domain is zero (Fig. 7c )
.
Finally, in ferrimagnetic materials (for example, ferrites) there is both parallel and antiparallel ordering (Fig. 7, d
), which results in weak magnetism.
There are two convincing experimental confirmations of the existence of domains. The first of them is the so-called Barkhausen effect, the second is the method of powder figures. In 1919, G. Barkhausen established that when an external field is applied to a sample of ferromagnetic material, its magnetization changes in small discrete portions. From the point of view of domain theory, this is nothing more than an abrupt advance of the interdomain wall, encountering on its way individual defects that delay it. This effect is usually detected using a coil in which a ferromagnetic rod or wire is placed. If you alternately bring a strong magnet towards and away from the sample, the sample will be magnetized and remagnetized. Abrupt changes in the magnetization of the sample change the magnetic flux through the coil, and an induction current is excited in it. The voltage generated in the coil is amplified and fed to the input of a pair of acoustic headphones. Clicks heard through headphones indicate an abrupt change in magnetization.
To reveal the domain structure of a magnet using the powder figure method, a drop of a colloidal suspension of ferromagnetic powder (usually Fe3O4) is applied to a well-polished surface of a magnetized material. Powder particles settle mainly in places of maximum inhomogeneity of the magnetic field - at the boundaries of domains. This structure can be studied under a microscope. A method based on the passage of polarized light through a transparent ferromagnetic material has also been proposed.
Weiss's original theory of magnetism in its main features has retained its significance to this day, having, however, received an updated interpretation based on the idea of uncompensated electron spins as a factor determining atomic magnetism. The hypothesis about the existence of an electron’s own momentum was put forward in 1926 by S. Goudsmit and J. Uhlenbeck, and at present it is electrons as spin carriers that are considered “elementary magnets”.
To explain this concept, consider (Fig. a free atom of iron, a typical ferromagnetic material. Its two shells ( K
and
L
), those closest to the nucleus, are filled with electrons, with the first of them containing two and the second containing eight electrons.
In the K
shell, the spin of one of the electrons is positive and the other is negative.
In the L
shell (more precisely, in its two subshells), four of the eight electrons have positive spins, and the other four have negative spins.
In both cases, the electron spins within one shell are completely compensated, so that the total magnetic moment is zero. In the M
shell the situation is different, since out of the six electrons located in the third subshell, five electrons have spins directed in one direction, and only the sixth in the other.
As a result, four uncompensated spins remain, which determines the magnetic properties of the iron atom. (There are only two valence electrons in the outer N
shell, which do not contribute to the magnetism of the iron atom.) The magnetism of other ferromagnets, such as nickel and cobalt, is explained in a similar way. Since neighboring atoms in an iron sample strongly interact with each other, and their electrons are partially collectivized, this explanation should be considered only as a visual, but very simplified diagram of the real situation.
The theory of atomic magnetism, based on taking into account the electron spin, is supported by two interesting gyromagnetic experiments, one of which was carried out by A. Einstein and W. de Haas, and the other by S. Barnett. In the first of these experiments, a cylinder of ferromagnetic material was suspended as shown in Fig. 9. If current is passed through the winding wire, the cylinder rotates around its axis. When the direction of the current (and therefore the magnetic field) changes, it turns in the opposite direction. In both cases, the rotation of the cylinder is due to the ordering of the electron spins. In Barnett's experiment, on the contrary, a suspended cylinder, sharply brought into a state of rotation, becomes magnetized in the absence of a magnetic field. This effect is explained by the fact that when the magnet rotates, a gyroscopic moment is created, which tends to rotate the spin moments in the direction of its own axis of rotation.
For a more complete explanation of the nature and origin of short-range forces that order neighboring atomic magnets and counteract the disordering influence of thermal motion, one should turn to quantum mechanics. A quantum mechanical explanation of the nature of these forces was proposed in 1928 by W. Heisenberg, who postulated the existence of exchange interactions between neighboring atoms. Later, G. Bethe and J. Slater showed that exchange forces increase significantly with decreasing distance between atoms, but upon reaching a certain minimum interatomic distance they drop to zero.
The essence
The magnetic properties of materials are mainly due to the magnetic moments of the orbital electrons of their atoms. The magnetic moments of atomic nuclei are typically thousands of times smaller than those of electrons, and are therefore insignificant in the context of magnetizing materials. Nuclear magnetic moments are nevertheless very important in other contexts, especially in nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI).
Typically, a huge number of electrons in a material are arranged in such a way that their magnetic moments (both orbital and internal) are reduced to nothing. This is due to some extent to the fact that electrons pair up with opposite intrinsic magnetic moments as a result of the Pauli principle (see electron configuration) and combine into filled subshells with zero net orbital motion.
In both cases, electrons preferentially use circuits in which the magnetic moment of each electron is canceled out by the opposite moment of the other electron. Moreover, even when the electron configuration is such that there are unpaired electrons and/or unfilled subshells, it is often the case that different electrons in a solid will contribute magnetic moments that point in different, random directions, so that the material will not be magnetic.
Sometimes, either spontaneously or due to an applied external magnetic field, each of the electrons' magnetic moments will, on average, line up. The right material can then create a strong, net magnetic field.
The magnetic behavior of a material depends on its structure, in particular on its electronic configuration, for the reasons given above, as well as on temperature. At high temperatures, random thermal motion makes it difficult for electrons to align.
Magnetic materials, their properties, application, classification
To create elements and devices of control and automation systems, magnetic materials , which mainly meet the following requirements:
1. The material should be easily magnetized under the action of a constant field or a unipolar field pulse and easily remagnetized in an alternating field; the hysteresis loop should be quite narrow with a small value of H C and a large value of m. Such requirements make it possible to increase the sensitivity of electromagnetic elements.
2. The materials must have a high saturation induction value B S, i.e. ensure the penetration of a large magnetic flux into a core with an appropriate cross-section. Fulfilling this requirement allows us to obtain the smallest dimensions and weight of the device, and if the dimensions are specified, then the greatest power or voltage at the output of the device.
3. When working in an alternating magnetic field, the material should have the lowest costs, which form eddy currents, magnetic viscosity and hysteresis, because they determine the operating temperature of the core and device. Reducing them not only increases the efficiency of the device, but also makes it possible to create elements that operate at higher frequencies (400, 500, 1000 Hz and more) and have significantly higher speed and smaller dimensions and weight than elements that are powered by an industrial frequency voltage of 50 Hz .
In addition to the listed basic requirements for magnetic materials used in certain electromagnetic devices, specific requirements are set.
Thus, to improve temperature stability (consistency of magnetic properties when the ambient temperature changes), it is important that the Curie point of the material is as high as possible.
The closer to unity the squareness coefficient of the material is, the linear dependence of the output signal on the input signal is, the easier it is to recognize signals in digital devices.
The clearly discovered magnetic anisotropy improves the quality of devices based on thin magnetic films, and the high purity of the crystalline structure of the material is a necessary condition for creating devices based on cylindrical magnetic domains.
Magnetic materials can be divided into hard magnetic materials , for which the intensity Hc is tens and hundreds of amperes per centimeter, and soft magnetic materials with a tension Hc of tenths and hundredths of an ampere per centimeter. Hard magnetic materials are used for the manufacture of permanent magnets, soft magnetic materials are used for the manufacture of elements in which the field is created by currents passing through the windings.
To create elements and devices of control systems, mainly soft magnetic materials . Magnetic-hard powder materials are included in ferolacs that cover magnetic tapes and disks.
Soft magnetic materials can be divided into three groups: electrical steels, alloys based on iron with other metals (nickel, cobalt, aluminum) and ferrites (non-metallic ferromagnets).
Electrical steels are the cheapest materials, having high saturation inductions (about 1.8 ... 2.3 T), and this makes it possible to create compact and cheap electromagnetic elements from them. But due to the relatively large (compared to iron-nickel alloys) coercive force of electrical steel (about 0.1 ¸ 0.5 A / cm), the sensitivity of steel elements to changes in the external field generated by the windings is low.
Zalizonickel alloys (permalloy) are 15-20 times more expensive than steel alloys, have a lower saturation induction, but make it possible to obtain highly sensitive magnetic elements due to their low coercive force and high initial magnetic permeability. Zalizonickel alloys are manufactured in the form of sheets or strips. The thickness of the tape sometimes reaches several micrometers.[adsense_id=”1″]
Zalizoaluminium alloys 16YUKH and 16YUM, which contain 16% aluminum, are not inferior in magnetic properties to permalloy, but have increased (10 ... 20 times more than in permalloy) wear resistance. They are widely used for the manufacture of magnetic heads in magnetic recording devices, where during operation the head continuously rubs against the surface of the tape.
Ferrites are non-metallic magnetic materials (solid solutions) made from a mixture of iron oxides with oxides of magnesium, copper, manganese, nickel and other metals. The general formula of ferrites is MeO × Fe2 Oz, where Me is any metal.
The oxides are crushed into small pieces and mixed in a certain proportion. Magnetic cores of the required sizes and configurations are pressed from the resulting mixture at a pressure of 10-30 kN/cm2 (1-3 t/cm2) and burned at a temperature of 1200-1400 ° C. The finished gray-black magnetic cores have high hardness, but are quite fragile . The windings are usually wound directly onto ferrite magnetic cores without additional insulation of the latter. The electrical resistivity of ferrites is millions of times greater than that of metal ferromagnets, which practically eliminates eddy currents. This allows magnetization reversal ferrites with a frequency of hundreds of kilohertz and ensure high speed of operations of modern control and computing machines. The most common magnesium-manganese ferrites are VT grades (1.3VT, 0.16 VT, etc.). They have a relatively low Curie point (140 - 300 ° C), which causes a significant change in their magnetic parameters when heated. Lithium-based ferrites, with a Curie point of 630 ° C, have significantly better temperature characteristics. Biferites are widely used for magnetic circuits of digital devices; there are ferrites with two metals, for example, magnesium-manganese or lithium-sodium ferrites, as well as polypherites, which are solid solutions of three or more ferrites.
Magnetic hard materials. Magnetic-hard materials, as already noted, are used:
— For the manufacture of permanent magnets;
— To record information (for example, for sound recording).
When assessing the properties of magnetically hard materials, mechanical properties (strength), workability of the material during the production process, as well as density, electrical resistivity, cost, etc. may be significant. In some cases, the issue of stability of magnetic properties is especially important.
The most important materials for permanent magnets are Fe-Ni-Al alloys. The mechanism of dispersion hardening plays a major role in the formation of the highly coercive state of these alloys.
Such materials have a high coercivity value, because their magnetization occurs mainly due to rotation processes.[adsense_id=»1″]
Fe-Ni-Al alloys without alloying elements are not used due to their relatively low magnetic properties. The most common alloys are those alloyed with copper and cobalt. High-cobalt alloys containing more than 15% Co are typically used with a magnetic or magnetic and crystalline texture.
The magnetic texture is the result of thermomagnetic treatment, which consists of cooling the alloy in a magnetic field with a strength of 160-280 kA/m from high temperatures (1250-1300 0 C) to approximately 500 0 C. In this case, an increase in magnetic characteristics occurs only in the direction of the field action, those. the material becomes magnetically anisotropic.
A further significant increase in the magnetic properties of Fe-Ni-Al-(Co) alloys is possible by creating magnets from a macrostructure in the form of columnar crystals. The crystalline structure is obtained through special cooling conditions of the alloy.
Here are brief recommendations for choosing alloy grades. Cobalt-free alloys (UND, etc.). There are cheap ones, their properties are relatively low. Alloys YUNDK15 and YUNDK18 are used when relatively high magnetic properties are required and the material should not have magnetic anisotropy. Alloys containing 24% Co (YuN13DK24, etc.) have high magnetic properties in the direction of the magnetic texture, are well technologically developed and are widely used.
Alloys with directional crystallization, for example YuN13DK25BA, etc., which have the highest W max and, therefore, can provide the smallest mass and dimensions of magnetic systems.
In cases where the system is open, alloys with the highest Hc are used, for example titanium alloy YUNDK35T5.
Alloys with a single-crystal structure (YUNDK35T5AA and YUNDK40T8AA) have the following advantages compared to alloys with directional crystallization: higher magnetic properties due to further improvement of the structure, the presence of three mutually perpendicular directions in which the properties are optimal; better mechanical properties.
The main disadvantages of Fe-Ni-Al-(Co) alloys are poor mechanical properties (high hardness and brittleness), which significantly complicates their mechanical processing.
Powder magnets. Magnets produced by powder metallurgy methods can be divided into metal-ceramic, metal-plastic and oxide.
For the first two groups, the physical processes of the formation of a high-coercive state depend on the same reasons as for monolithic magnets; for the other two groups, a necessary condition for obtaining high-coercive properties is a state ground to a certain degree of dispersion, which corresponds to a single-domain structure.
Ceramic-metal magnets are produced from metal powders by pressing them without any material that binds them and sintering them at high temperatures. In terms of magnetic properties, they are only slightly inferior to cast magnets, but more expensive than others.
Metal-plastic magnets are produced, like metal-ceramic magnets, from metal powders, but they are pressed together with an insulating binder and heated to a low temperature necessary for the polymerization of the substance that binds them. Compared to cast magnets, they have reduced magnetic properties, but have high electrical resistance, low density and are relatively cheap.
Among oxidizing magnets, magnets based on barium and cobalt ferrites are of practical importance.
Barium magnets. The industry produces two groups of barium magnets: isotropic (BI) and anisotropic (BA).
Compared to cast magnets, barium magnets have a very high coercive force and low residual induction. The electrical resistivity r of barium magnets is millions of times higher than that of metallic materials, allowing barium magnets to be used in magnetic circuits that are exposed to high frequency fields. Barium magnets do not contain scarce and expensive materials; they are approximately 10 times cheaper than magnets with UNDC24.
The disadvantages of barium magnets include poor mechanical properties (high fragility and hardness) and, most importantly, a greater dependence of magnetic properties on temperature. The temperature coefficient of residual magnetic induction TC B r of barium magnets is approximately 10 times greater than TC B r of cast magnets. In addition, barium magnets have irreversible properties when cooled, i.e. have higher temperature stability than barium. However, they also have temperature hysteresis, but it does not appear in the region of negative temperatures, as in barium magnets, but at positive temperatures (when heated above 80 ° C).
Other materials for permanent magnets.
Martensitic steels. Martensite is the name given to the type of microstructure of steel obtained when it is hardened. The formation of martensite is accompanied by significant volumetric changes, the creation of large internal lattice stress and the appearance of large coercive force values.
Martensitic steels began to be used for the manufacture of permanent magnets earlier than other materials. Currently, they are used relatively little due to their low magnetic properties. However, they have not yet been completely abandoned, because they are inexpensive and can be machined on metal-cutting machines.
Alloys are plastically deformed. These alloys have high machinability properties. They are well stamped, cut with scissors, and processed on metal-cutting machines. Alloys that can be plastically deformed can be used to make tapes, plates, sheets, and wire. In some cases (when producing small magnets of complex configuration), it is advisable to use metal-ceramic technology. There are many grades of alloys that are plastically deformed, and the physical processes due to which they have high magnetic properties are varied. The most common alloys are kunife (Cu-Ni-Fe) and vikaloy (Co-V). Kunife alloys are anisotropic, magnetized in the rolling direction, and are often used in the form of thin wire and stamping. Vikaloy is used for the manufacture of the smallest magnets of complex or openwork configuration and as high-strength magnetic tapes or wire.
Alloys based on noble metals. These include alloys of silver with manganese and aluminum (silmanal) and alloys of platinum with iron (77.8% Pt; 22.2% Fe) or platinum with cobalt (76.7% Pt; 23.3% Co). Materials in this group, especially those containing platinum, are very expensive, so they are used only for subminiature magnets weighing a few milligrams. Metal-ceramic technology is widely used in the manufacture of magnets from all alloys of this group.
Elastic magnets. As noted, the most important disadvantage of the main groups of materials for permanent magnets - cast alloys and hard magnetic ferrites - is their poor mechanical properties (high hardness and brittleness). The use of plastically deformable alloys is limited by their high cost. Recently, rubber-based magnets have appeared. They can be of any shape that rubber technology allows - in the form of cords, long strips, sheets, etc. Such material is easily cut with scissors, stamped, bent, and twisted. It is known to use “magnetic rubber” as magnetic memory letters for computers, magnets for deflection systems in television, magnets for correcting, etc.
Elastic magnets are made of rubber and fine powder of hard magnetic materials (filler). Barium ferrite is most often used as a filler.
Materials for magnetic tapes. Magnetic tapes mean magnetic recording media. The most common are solid metal tapes made of stainless steel, bimetallic tapes and plastic-based tapes with a powder working layer. Solid metal tapes are used mainly for special purposes and when working in a wide temperature range; Plastic-based tapes are more widely used. The main purpose of a magnetic recording medium is to create a magnetic field on the surface of the reproduced head, the strength of which changes (as the tape is pulled) over time in the same way as the signal that is being recorded. The properties of tapes coated with magnetic powders significantly depend not only on the properties of the source materials, but also on the degree of particle grinding, the volumetric density of the magnetic material in the working layer, the orientation of the particles if they have shape anisotropy, etc.
The working layer (or thickness of the metal tape) should be as thin as possible, and the tape itself should be smooth and flexible to ensure maximum interaction (magnetic contact) between the magnetic materials of the tape and the head. The residual magnetization of the material should be as high as possible.
Contradictory requirements are placed on the coercive force: to reduce self-demagnetization, a higher possible value of H c is necessary (at least 24 kA / m), and to facilitate the process of erasing a record, a small H c is desirable. The requirements of high residual magnetization and minimal sensitivity to self-demagnetization are best satisfied with a rectangular section of the demagnetization hysteresis loop, i.e. It is desirable to have a maximum value of the convexity coefficient. Temperature and other changes in the magnetic properties of the tape material should be minimal.
The industry produces magnetic tapes made of non-rusting alloy EP-31A and bimetal EP-352/353. The tapes have a thickness of 0.005-0.01 mm, N c = 24 - 40 kA / m; B r = 0.08 T.
Domestic plastic-based tapes are made mainly of types A2601-6 (type 6 - for studio tape recorders) and A4402 - 6 (type 10 - for household and reportage). In accordance with GOST, the following is used in the designations of tapes: the first element - a letter index - indicates the purpose of the tape: A - sound recording, T - video recording, B - computer technology, I - exact recording: the second element - digital index (from 0 to 9), indicates the material bases: 2 - diacetylcellulose, 3 - triacetylcellulose, 4 - polyethylene terephthalag (lavsan), the third element is a digital index (from 0 to 9), means the thickness of the tape: 2 - 18 microns, 3 - 27 microns, 4 - 36 microns, 6 - 55 microns, 9 - more than 100 microns, the fourth element is a digital index (from 01 to 99), means the number of technological development; the fifth element is the numerical value of the nominal width of the tape in millimeters. After the fifth element there must be an additional letter index: P - for perforated tapes; P - for tapes used in radio broadcasting B - for tapes from household tape recorders.
The following materials are used for magnetic powders: iron ferrite (magnetite), cobalt ferrite, chromium dioxide, etc. Each of them has its own advantages and disadvantages. The most widely used is gamma iron oxide (g-Fe 2 O 3), which is needle-shaped with a particle length of about 0.4 μm and a length-to-diameter ratio of approximately three. Powder (g-Fe 2 O 3) is obtained by oxidizing magnetite (iron ferrite) FeO × Fe 2 O 3 by heating it in air at a temperature of about 150 o C.
The production of magnetic tapes can be varied. More often, the working layer (magnetic varnish) is applied to the finished base, for example, by pouring varnish from a die. Magnetic varnish is prepared in advance and consists of magnetic powder, a binder, a solvent, a plasticizer and various additives that promote wetting and separation of powder particles and reduce the abrasiveness of the working layer.
When using powders with anisotropy of particle shape (for example, needle-shaped g-Fe) in the production process of the tape, the lobes are oriented in a certain way as a result of the influence of a magnetic field on them. The final processing of the belt consists of calendering and polishing to improve the quality of its surface.
Type 6 tape provides high quality sound recording and playback when used in professional equipment at a speed of 19.05 cm/s and in household tape recorders at a speed of 9.53 and 4.75 cm/s.[adsense_id=»1″]
Tapes must be stored at a temperature of 10-25 ° C and a relative humidity of 50-60%; Temperatures above 30°C are unacceptable, temperatures below 10°C are not recommended.
In addition to types 6 and 10, the domestic industry produces other types of tapes, for example, the T4402-50 tape with a width of 50.8 mm for cross-line recording of black and white images.
Alloys based on rare earth metals (REM). A number of compounds and alloys with rare-earth metals have very high values of coercive force and maximum specific energy. Of this group of materials, the most interesting are intermetallic compounds of the RCo 5 type, where R is a rare earth metal.
In addition to the main groups of magnetic materials considered, some others are also used in technology, which have a limited scope of application.
Thermomagnetic materials. Thermomagnetic are materials with a significant dependence of magnetic induction (more precisely, saturation magnetization, because usually thermomagnetic material operates in saturation mode) on temperature in a certain range (in most cases +60 ¸ -60 0 C). Thermomagnetic materials are used mainly as magnetic shunts or additional supports. The inclusion of such elements in magnetic circuits makes it possible to compensate for temperature errors or to ensure a change in magnetic induction in the air gap according to a given law (thermal regulation).
Magnetostrictive materials. Magnetostriction has direct technical application in magnetostrictive vibrators (generators) of sound and ultrasonic vibrations, as well as in some radio circuits and devices (instead of quartz for frequency stabilization, in electromechanical filters, etc.).
Nickel, permendur (Fe-Co alloys characterized by high saturation magnetization), Alfer (Fe-Al alloys), nickel and nickel-cobalt ferrites, etc. are used as magnetostrictive materials.
Nickel has a large absolute value of the saturation magnetostriction coefficient l S = D l / l = -35 × 10 -6 (l is the length of the plate to the field, D l is the change in length as a result of the field; the minus sign means a decrease in length). Typically, grade H nickel is used with a thickness of 0.1 mm in the form of a rigid, unfired strip. After cutting, the plates are oxidized by heating in air to 800 o C for 15-25 minutes. The oxide film thus formed serves to electrically insulate the plates when making up the stack. Nickel has high anti-corrosion properties and a low temperature coefficient of elastic modulus.
Recently, magnetostrictive ferrites have been used more widely, especially in precision filters.
Alloys with high saturation induction. Of the common materials, iron has the highest induction (»2.1 T).
In cases where the highest requirements are put forward for the dimensions of the device, its mass and flow size, high-alcobalt alloys are used, in which the saturation induction reaches 2.43 T, which allows for savings in mass and volume compared to iron by 15 - 20% . In practice, alloys containing 30-51% Co and 1.5-2.0% V are used, which improves the technological properties of the alloys and the ability to process them in a cold state. These alloys are called permendur.
The saturation induction of alloys with high and low cobalt content is approximately the same. High-cobalt alloys in weak and medium fields have higher magnetic permeability values than low-cobalt alloys, but the latter are cheaper.[adsense_id=»1″]
In addition to its high saturation induction value, permendur has significant reversible permeability, which makes it especially valuable as a material for telephone membranes. Disadvantages of permendur: low electrical resistivity r, high cost and scarcity of cobalt and vanadium. Permendur is used in constant magnetic fields or in weak alternating fields with strong magnetization by a constant field. Of the materials in this group, the standardized alloy is 50 KF (49.0-51% Co; 1.5-2.0% V). The alloy has a saturation induction of at least 2.35 T and q = 980 °C.
The advantage of high-cobalt alloys over technically pure iron is felt at magnetic induction above 1.0 Tesla. The difference in magnetic permeability values reaches a maximum at a magnetic induction value of about 1.8 T, while the permeability of cobalt alloys is tens of times greater than the permeability of soft iron varieties.
Vasyura A.S. — Book “Elements and devices of automation control systems”
Liked this:
Like
Similar
Diamagnetism
Diamagnetism occurs in all materials and is the tendency of a material to resist an applied magnetic field and therefore be repelled by a magnetic field. However, in a material with paramagnetic properties (that is, with a tendency to amplify an external magnetic field), paramagnetic behavior dominates. Thus, despite its universal occurrence, diamagnetic behavior is only observed in purely diamagnetic material. There are no unpaired electrons in a diamagnetic material, so the electrons' own magnetic moments cannot create any volume effect.
Please note that this description is intended as a heuristic only. The Bohr-van Leeuwen theorem shows that diamagnetism is impossible according to classical physics, and that a proper understanding requires a quantum mechanical description.
Please note that all materials undergo this orbital response. However, in paramagnetic and ferromagnetic substances, the diamagnetic effect is suppressed by much stronger effects caused by unpaired electrons.
A paramagnetic material has unpaired electrons; that is, atomic or molecular orbitals with exactly one electron in them. While the Pauli exclusion principle requires paired electrons to have their own ("spin") magnetic moments pointing in opposite directions, causing their magnetic fields to cancel out, an unpaired electron can align its magnetic moment in either direction. When an external field is applied, these moments will tend to align in the same direction as the applied field, enhancing it.
The concept of hysteresis. Permanent magnetism
Ferromagnetic and ferrimagnetic materials have the property of residual magnetization. This property is due to the phenomenon of hysteresis - delay. Its essence is that the change in the magnetization of the material lags behind the change in the external field. If, upon reaching saturation, the field strength is reduced, the magnetization will change not in accordance with the magnetization curve, but in a more gentle manner, since a significant part of the domains remains oriented according to the field vector. Thanks to this phenomenon, permanent magnets exist.
Demagnetization occurs when the direction of the field changes, when it reaches a certain value called the coercive (retention) force. The greater its value, the better the substance retains residual magnetization. The hysteresis loop closes with the next change in voltage in direction and magnitude.
Ferromagnets
A ferromagnet, like a paramagnetic substance, has unpaired electrons. However, in addition to the tendency for the electrons' intrinsic magnetic moment to be parallel to the applied field, in these materials there is also a tendency for these magnetic moments to orient parallel to each other in order to maintain a state of reduced energy. Thus, even in the absence of an applied field, the magnetic moments of the electrons in the material spontaneously line up parallel to each other.
Each ferromagnetic substance has its own individual temperature, called the Curie temperature, or the Curie point, above which it loses its ferromagnetic properties. This is because the thermal tendency towards disorder suppresses the energy reduction due to ferromagnetic order.
You may be interested in:Top 10 most correct translators
Ferromagnetism occurs only in a few substances; Commonly used are iron, nickel, cobalt, their alloys and some alloys of rare earth metals.
The magnetic moments of the atoms in a ferromagnetic material cause them to behave like tiny permanent magnets. They clump together and organize into small regions of more or less uniform alignment called magnetic or Weiss domains. Magnetic domains can be observed using a magnetic force microscope to reveal the boundaries of the magnetic domains, which resemble the white lines in the sketch. There are many scientific experiments that can physically show magnetic fields.
Paramagnets
Substances belonging to this group are characterized by positive magnetic susceptibility (very low, about 10-5 – 10-6). They are magnetized parallel to the vector of the applied field, that is, they are drawn into it, but the interaction of paramagnetic materials with it is very weak, like that of diamagnetic materials. Their magnetic permeability is close to the permeability of vacuum, only slightly exceeding it.
In the absence of an external field, paramagnets, as a rule, do not have magnetization: their atoms have their own magnetic moments, but they are oriented randomly due to thermal vibrations. At low temperatures, paramagnetic materials can have a small intrinsic magnetization, which strongly depends on external influences. However, the influence of thermal motion is too great, as a result of which the elementary magnetic moments of paramagnets are never set exactly in the direction of the field. This is the reason for their low magnetic susceptibility.
The forces of interatomic and intermolecular interaction also play a significant role, promoting or, on the contrary, resisting the ordering of elementary magnetic moments. This causes a wide variety of magnetic properties of paramagnetic substances.
This group of substances includes many metals, such as tungsten, aluminum, manganese, sodium, and magnesium. Oxygen, iron salts, and some oxides are paramagnetic.
The Role of Domains
When a domain contains too many molecules, it becomes unstable and splits into two domains aligned in opposite directions so that they stick together more stably, as shown on the right.
When exposed to a magnetic field, domain boundaries move so that domains aligned with the magnetic field grow and dominate the structure (dashed yellow area), as shown on the left. When the magnetizing field is removed, the domains may not return to their unmagnetized state. This causes the ferromagnetic material to become magnetized, forming a permanent magnet.
When magnetization is strong enough so that the dominant domain overlaps all others, resulting in the formation of only one distinct domain, the material becomes magnetically saturated. When a magnetized ferromagnetic material is heated to the Curie point temperature, the molecules are mixed to such an extent that the magnetic domains become disorganized and the magnetic properties they cause cease. As the material cools, this domain alignment structure spontaneously returns, much in the same way that a liquid can freeze into a crystalline solid.
Diamagnets
Due to some structural features of electron clouds, atoms (or molecules) of diamagnetic materials do not have a magnetic moment. It appears when an external field appears. The induced, induced field has the opposite direction, and the resulting field turns out to be somewhat weaker than the external one. True, this difference cannot be significant.
The magnetic susceptibility of diamagnetic materials is expressed by negative numbers with an order of magnitude from 10-4 to 10-6 and does not depend on the field strength; magnetic permeability is lower than that of vacuum by the same order of magnitude.
The application of a non-uniform magnetic field leads to the fact that the diamagnetic material is pushed out by this field, as it tends to shift to a region where the field is weaker. The effect of diamagnetic levitation is based on this feature of the magnetic properties of substances of this group.
Diamagnets represent a wide group of substances. It includes metals such as copper, zinc, gold, silver, and bismuth. It also includes silicon, germanium, phosphorus, nitrogen, hydrogen, and inert gases. Complex substances include water, many salts, and organic compounds. Ideal diamagnetic materials are superconductors. Their magnetic permeability is zero. The field cannot penetrate into the superconductor.
Antiferromagnet
In an antiferromagnet, unlike a ferromagnet, the intrinsic magnetic moments of neighboring valence electrons tend to point in opposite directions. When all the atoms in a substance are arranged so that each neighbor is antiparallel, the substance is antiferromagnetic. Antiferromagnets have zero net magnetic moment, which means they do not produce a field.
Antiferromagnets are rare compared to other types of behavior and are most often observed at low temperatures. At different temperatures, antiferromagnets exhibit diamagnetic and ferromagnetic properties.
In some materials, neighboring electrons prefer to point in opposite directions, but there is no geometric arrangement in which each pair of neighbors is anti-aligned. This is called spin glass and is an example of geometric frustration.
Structure of matter and magnetism
The first theory explaining the nature of magnetism through the relationship of electrical and magnetic phenomena was created by the French physicist J.-M. Ampere in the 20s of the 19th century. Within the framework of this theory, Ampere assumed the presence in physical bodies of microscopic closed currents, usually compensating each other. But in substances that have magnetic properties, such “molecular currents” create a surface current, causing the material to become a permanent magnet. This hypothesis has not been confirmed, with the exception of one very important idea - about microcurrents as sources of magnetic fields.
Microcurrents in matter actually exist due to the movement of electrons in atoms and create a magnetic moment. In addition, electrons have their own magnetic moment of a quantum nature.
The total magnetic moment of a substance, that is, the totality of elementary currents in it, in relation to a unit volume, determines the state of magnetization of a macroscopic body. In most substances, the particle moments are oriented disorderly (the leading role in this is played by thermal chaotic vibrations), and the magnetization is practically zero.
Magnetic properties of ferromagnetic materials
Like ferromagnetism, ferrimagnets retain their magnetization in the absence of a field. However, like antiferromagnets, adjacent pairs of electron spins tend to point in opposite directions. These two properties do not contradict each other because, in an optimal geometric arrangement, the magnetic moment from a sublattice of electrons that points in one direction is greater than that from a sublattice that points in the opposite direction.
Most ferrites are ferrimagnetic. The magnetic properties of ferromagnetic materials are considered indisputable today. The first magnetic substance discovered, magnetite, is a ferrite and was originally thought to be ferromagnetic. However, Louis Néel refuted this by discovering ferrimagnetism.
When a ferromagnet or ferrimagnet is small enough, it acts as a single magnetic spin that is subject to Brownian motion. Its response to a magnetic field is qualitatively similar to that of a paramagnetic, but much larger.
A little more about the use of magnetic materials
Modern high-tech production requires the use of magnets made from structural materials, including composite materials, with specified magnetic properties of substances. These are, for example, ferromagnetic-superconductor or ferromagnetic-paramagnetic magnetic nanocomposites used in spintronics, or magnetopolymers - gels, elastomers, latexes, ferrofluids, which are widely used.
Various magnetic alloys are also extremely in demand. The neodymium-iron-boron alloy is characterized by high resistance to demagnetization and power: the neodymium magnets mentioned above, being the most powerful permanent magnets to date, are used in a wide variety of industries, despite the presence of some disadvantages, such as fragility. They are used in magnetic resonance imaging scanners, wind generators, when cleaning technical fluids and lifting heavy loads.
Very interesting are the prospects for using antiferromagnets in low-temperature nanostructures for the manufacture of memory cells, which can significantly increase the recording density without disturbing the state of neighboring bits.
It must be assumed that the use of the magnetic properties of substances with given characteristics will increasingly expand and provide serious technological breakthroughs in various fields.
Electromagnets
An electromagnet is a magnet in which a magnetic field is created by an electric current. The magnetic field disappears when the current is turned off. Electromagnets typically consist of many closely spaced turns of wire that create a magnetic field. Wire turns are often wound around a magnetic core made of ferromagnetic or ferrimagnetic material such as iron; The magnetic core concentrates the magnetic flux and creates a more powerful magnet.
The main advantage of an electromagnet over a permanent magnet is that the magnetic field can be quickly changed by controlling the amount of electric current in the winding. However, unlike a permanent magnet, which requires no power, an electromagnet requires a continuous supply of current to maintain the magnetic field.
Electromagnets are widely used as components in other electrical devices such as motors, generators, relays, solenoids, loudspeakers, hard drives, MRI machines, scientific instruments, and magnetic separation equipment. Electromagnets are also used in industry to pick up and move heavy iron objects such as scrap metal and steel. Electromagnetism was discovered in 1820. At the same time, the first classification of materials according to magnetic properties was published.
Source
Hard magnetic materials
Hard magnetic materials are used to make permanent magnets. These materials must meet the following requirements:
- have a large residual induction;
- have a high maximum magnetic energy;
- have stable magnetic properties.
The cheapest material for permanent magnets is carbon steel (0.4 - 1.7% carbon, the rest is iron). Magnets made of carbon steel have low magnetic properties and quickly lose them under the influence of heat, shock and shock.
Alloy steels have better magnetic properties and are used for the manufacture of permanent magnets more often than carbon steel. These steels include chromium, tungsten, cobalt and cobalt-molybdenum.
For the manufacture of permanent magnets, alloys based on iron - nickel - aluminum have been developed in technology. These alloys are characterized by high hardness and brittleness, so they can only be processed by grinding. The alloys have exceptionally high magnetic properties and high magnetic energy per unit volume.
Table 1 shows data on the composition of some hard magnetic materials for the manufacture of permanent magnets.
Table 1
Chemical composition of magnetically hard materials
| Name of material | Chemical composition in weight percent | Relative weight per unit magnetic energy |
| Carbon steel Chromium steel Tungsten steel Cobalt steel Cobalt-molybdenum steel Alni Alnisi Alnico Magnico | 0.45 C rest Fe 2 – 3 Cr; 1 C 5 W; 1 C 5 – 30 Co; 5 – 8 Cr; 1.5 – 5 W 13 – 17 Mo; 10 – 12 Co 12.5 Al; 25 Ni; 5 CH 14 Al; 34 Ni; 1 Si 10 Al; 17 Ni; 12Co; 6 CH 24 Co; 13 Si; 8 Al; 3 Dc | 26,7 17,2 15,8 5,1 – 12,6 3,8 3,6 3,4 3,1 1 |