The use of carbon steels is widespread in construction and industry. The group of so-called technical iron has many advantages that lead to increased performance qualities of final products and structures. Along with optimal characteristics of strength and load resistance, such alloys are also distinguished by flexible dynamic properties. In particular, hypoeutectoid steel, which also contains a considerable percentage of carbon mixtures, is valued for its high ductility. But this is not all the advantages of this type of high-strength iron.
Hypoeutectoid and eutectoid steels
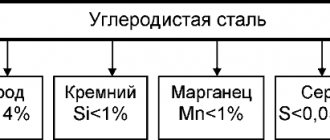
Steels containing from 0.025 to 0.8% carbon are called hypoeutectoid
.
The structure of these steels consists of ferrite (light background) and pearlite (dark grains). The amount of pearlite increases and ferrite decreases in proportion to the increase in carbon content (Fig. 5) in accordance with the phase diagram (Fig. 1).
| A | b | V |
| Ferrite + pearlite – a + (a+Fe3C) – 0.2-0.3% C | Pearlite + ferrite – (a+Fe3C)+ a – 0.4-0.5% C | Pearlite + ferrite – (a+Fe3C) + a 0.5-0.7% C |
Fig.5. Microstructure of hypoeutectoid steels:
A
– steel 20,
b
– steel 45,
c
– steel 60
Therefore, considering that ferrite practically does not dissolve carbon, and the presence of 100% pearlite in the structure corresponds to 0.81% C, we can find the carbon content
in any hypoeutectoid steel, using a microscope to determine the quantitative relationship between the structural components and then solving a simple proportion.
where A is the amount of perlite, determined visually using a microscope.
With a content of 0.8% C, the steel is called eutectoid
and consists of only perlite.
The hardness and tensile strength of eutectoid steel is higher than that of hypoeutectid steel, and its ductility is lower.
Hypereutectoid steels
Steels with a carbon content of 0.81 to 2% are called hypereutectoid.
, their structure consists of
perlite and secondary cementite
.
Cementite is the most fragile and hard (HB>800) structural component. The plasticity of cementite is negligible and practically equal to zero, which is probably a consequence of the complex structure of its crystal lattice. The crystal structure of cementite is very complex. There are many different ways to depict it, one of the most successful is shown in Fig. 6.
The cementite mesh in the steel structure reduces its ductility and increases its hardness. Therefore, with an increase in the amount of secondary cementite, in proportion to the increase in the concentration of carbon in it, its hardness increases, and its ductility decreases.
Rice. 6. Crystal structure of cementite
Cementite contains 6.67% carbon and is the most brittle and hard (HB up to 800) structural component of iron-carbon alloys.
In hypereutectoid steel, secondary cementite is usually located in the form of a light network or light grains (chains) along the boundaries of pearlite grains or in the form of needles (Fig. 7).
Fig.7. Microstructure of hypereutectoid steel U12 - 1.2% C
(perlite + secondary cementite)
a – secondary granular cementite; b – in the form of a grid along grain boundaries
In steels containing slightly less than 0.81% carbon, ferrite can also precipitate in the form of a network along the boundaries of pearlite grains. With conventional etching with a 4% nitric acid solution, this mesh also turns out to be light. To determine whether this mesh is ferritic or cementite, a microsection is etched with sodium picrate.
If the mesh remains light after etching, then it is ferrite and, therefore, the steel is hypoeutectoid; if the mesh darkens, then it is cementite, and the steel is hypereutectoid.
Secondary cementite in hypereutectoid steel occupies a small area, which is difficult to determine by eye. Therefore, the method that determines the carbon content in hypoeutectoid steels is not used for hypereutectoid steels.
The separation of secondary cementite along the grain boundaries of austenite and perlite cementite in the form of plates is undesirable, since such a structure has increased fragility, is poorly processed by cutting, and after final heat treatment the finished parts (tools) will have reduced mechanical properties, mainly low ductility and impact strength. Therefore, they strive to obtain cementite in the form of small rounded grains (balls). The structure of granular pearlite is the initial structure for tool steels (Fig. 4).
Thus, the properties of steel after slow cooling are determined by the properties of its structural components and their quantitative ratio. The structure of steel consists of pearlite with excess either ferrite or cementite, depending on the amount of carbon in it. Consequently, it is the carbon content in steel that determines its mechanical and technological properties - strength, hardness, ductility, toughness.
The amount of cementite in the steel structure increases in direct proportion to the carbon content, and as mentioned above, the hardness of cementite HB>800 (8000-8500 MPa) is an order of magnitude greater than the hardness of ferrite HB 45-80 (450-800 MPa). In addition, cementite particles increase the resistance to dislocation movement, i.e. increase resistance to deformation, reduce ductility and viscosity. As a result, with an increase in carbon content in steel to 1.0%, hardness, strength, and yield strength increase and plasticity indicators (relative elongation and contraction) and impact strength decrease (Fig. 6).
When the carbon content is above 1.0-1.1%, the hardness of the steel in the annealed state increases, and the strength decreases due to the presence of secondary cementite, which forms a continuous network and causes brittle premature failure.
With an increase in carbon content, the structure of the steel changes, the amount of cementite increases and the amount of ferrite decreases. This leads to a corresponding change in the properties of steel.
Fig. 8. Effect of carbon on the mechanical properties of steel
The more carbon in steel, the higher the hardness and strength, but the lower the ductility
(Fig. 8).
The mechanical properties of steel also depend on the shape and size of the ferrite-cementite mixture.
The more dispersed (thinner) the particles of the ferrite-cementite mixture are, the higher the hardness and strength of the steel.
The granular form of cementite, compared to the lamellar form, with the same hardness, has
higher ductility and impact strength .
With increasing carbon content
in steel:
- weldability is reduced, carbon also contributes to the formation of cracks and pores in the weld seam during welding,
Cementite: forms of existence
This is the name given to the compound of carbon and iron. It is a component of cast iron and some steels. It contains 6.67% carbon.
Its crystal includes several octahedra; they are located with respect to each other at a certain angle. Inside each of them is a carbon atom. As a result of this construction, the following picture is obtained - one atom comes into contact with several atoms of iron, and iron, in turn, is connected with three atoms of this element.
Crystal lattice of cementite
This substance has all the properties that are inherent in metals - electrical conductivity, a peculiar shine, high thermal conductivity. That is, a mixture of iron and carbon behaves like a metal. This material has a certain fragility. Most of its properties are determined by the complex structure of the crystal lattice.
This material melts at 1600 degrees Celsius. But there are several opinions on this matter; some researchers believe that its melting point lies in the range from 1200 to 1450, others determine that the upper level is 1300 °C.
Primary cementite
Metallurgists distinguish three types of this substance - primary, secondary, tertiary.
Iron-cementite diagram
Primary, obtained from the liquid during hardening of alloys that contain 5.5% carbon. The primary one has the shape of large plates.
Secondary
This element is obtained from austenite when the latter is cooled. On the diagram, this process can be seen in the Fe – C diagram. Cementite is presented in the form of a grid placed along the grain boundaries.
Tertiary
This type is derived from ferrite. It is shaped like needles.
There are other forms of cementite in metallurgy, for example, Stead's cementite, etc.
Other structural components in the iron-carbon system
Perlite
Perlite is a mechanical mixture that consists of ferrite and cementite. Ledeburite is a variable solution.
Perlite
At temperatures from 1130 to 723 ° C, its composition includes austenite and cementite. At lower temperatures it consists of austenite replacing ferrite.
Structure - eutectoid steel
The structure of eutectoid steel consists of pearlite. [1]
The structure of eutectoid steel in its extreme states - equilibrium (pearlite) and nonequilibrium (austenitic) - is known; in the first, a clearly defined lamellar pearlite is obtained (Fig. [2]
From examining the structures of these three types of cast iron, we can conclude that their metal base is similar to the structure of eutectoid steel, hypoeutectoid steel and iron. Consequently, the structure of cast iron differs from steel in that cast iron contains graphite inclusions, which predetermine the specific properties of cast iron. [3]
From examining the structures of these three types of cast iron, we can conclude that their metal base is similar to the structure of eutectoid steel, hypoeutectoid steel and iron. [5]
From an examination of the structures of these three types of cast iron, we can conclude that their metal base is similar to the structure of eutectoid steel, hypoeutectoid steel and iron. [7]
The transformations occurring in alloys of iron and carbon are reversible. If the structure of eutectoid steel (0 8% C) upon cooling below 723 C transforms from austenite to pearlite, then during heating at 723 C the reverse transformation will occur - pearlite to austenite. Structural transformations in pre- and hypereutectoid steels occur in the reverse order upon heating. [8]
It follows from the diagram that the properties of sorbitol and trostite occupy an intermediate position between the properties of pearlite and martensite. The properties of the structures of hypoeutectoid and hypereutectoid steels differ from the properties of the same structures of eutectoid steel depending on the C content. [10]
Steel with 0 8% C containing only eutectoid is called eutectoid steel. The eutectoid was given a special name - pearlite. The structure of eutectoid steel is shown in Fig. It consists of one perlite; in this case, the entire field of the thin section is filled with perlite. [eleven]
CC (below the upper critical point Ac3), and cooled at a rate exceeding the critical VK. Incomplete hardening is used for eutectoid and hypereutectoid carbon steels. The initial structure of hypereutectoid steel consists of pearlite and secondary cementite. When heated above Ag, pearlite transforms into austenite (PA), and cementite remains undissolved. With rapid cooling, the A - M transformation occurs, and as a result, the structure of hypereutectoid steel consists of martensite, cementite and retained austenite. The presence of cementite in the structure increases the hardness and wear resistance of steel. The structure of hardened eutectoid steel consists of martensite and retained austenite. [12]
To better understand the Fe-C diagram, let us trace the cooling process of a hypoeutectoid alloy of composition / (Fig. As it cools at point a, the alloy begins to solidify, and austenite crystals fall out of the liquid alloy. Between points a and b, the amount of liquid will gradually decrease, and at point b the alloy will finally harden, obtaining the austenite structure. This is where primary crystallization ends. It cools down to the point in austenite without any changes. At point b on the GS line, secondary crystallization will begin, associated with the separation of ferrite from austenite and the transition of y-iron to a-iron The release of free ferrite from the solid solution leads to an increase in the amount of carbon in the remaining austenite; at point g the carbon content is 0-8% and austenite, which has a eutectoid concentration, decomposes to form pearlite. Below point g the alloy does not undergo any changes upon further cooling. Alloys containing 0 045 - 1 45% carbon are classified as steels. Steels containing less than 0 8% carbon will cool similarly to the alloy considered, and the structure of such steels during slow cooling will consist of ferrite and pearlite. The structure of eutectoid steel consists of pearlite, and hypereutectoid steel consists of pearlite and cementite. [13]
Source
Components in the iron-carbon system
Austenite
The atoms are placed in a face-centered cell. The hardness of austenite has a hardness of 200 ... 250 Brinell units. In addition, it has good ductility and is paramagnetic.
Iron
Iron is a material classified as a metal. Its natural color is silver-gray. In its pure form it is very plastic. Its specific gravity is 7.86 g/cc. cm. Melting point is 1539 °C. In practice, industrial iron is most often used, which contains the following impurities - manganese, silicon and many others. The mass fraction of impurities does not exceed 0.1%.
Iron
Iron has such a property as polyformism. That is, with the same chemical composition, this substance can have a different crystal lattice structure and, accordingly, different properties. Modifications of iron are called respectively - B, D, D. All these modifications exist under different conditions. For example, type B can only exist at a temperature of 911 °C. Type G can exist in the range from 911 to 1392 °C. Type D exists in the range from 1392 to 1539 °C.
Each type has its own crystal lattice shape, for example, type B has a cube-shaped lattice, type G lattice has a face-centered cubic shape. The D-type lattice has the shape of a volume-centered cube.
Another property is that at temperatures below 768, iron is ferrimagnetic, and as it increases, this property is lost.
The points of polymorphic and magnetic transformation are called critical. On the table they are designated as follows - A2, A3, A4. Digital indices indicate the type of transformation. To more fully distinguish the transformation of iron from one type to another, the indices c and r are added to the designation. The first one talks about heating, the second one talks about cooling.
Iron polymorphs
With high ductility parameters, iron does not have high hardness; on the Brinell scale it is equal to 80 units.
Iron has the ability to form solid solutions. They can be divided into two groups - substitution and implementation solutions. The former consist of iron and other metals, the latter of iron and carbon, hydrogen and nitrogen.
Carbon
Another component of the system is carbon. It is a non-metal and has three modifications in the form of diamond, graphite and coal. It melts at 3500 °C.
Allotropic modifications of carbon
In an iron alloy, this element is found in the form of a solid solution, it is called cementite or in the form of graphite. In this form it is present in gray cast iron. Graphite is neither ductile nor durable.
Cementite
The carbon share is 6.67%. It has high hardness - 800 HB, but at the same time it lacks ductility. Does not have polymorphic properties.
It has the following property - when forming a substitution solution, carbon can be replaced by atoms of other substances, for example, chromium or nickel. This solution is called a doped solution.
Cementite
It is not stable; under certain conditions it can decompose, and carbon is transformed into graphite. This property has found application in the formation of cast irons.
By the way, in a liquid state, iron can dissolve impurities in itself, while forming a homogeneous mass.
Ferrite
This is the name given to the solid solution in which carbon is introduced into iron.
It dissolves with a certain variability; at normal (room) temperature, the volume of carbon is within 0.006%; at 727 °C, the carbon concentration will be 0.02%. Upon reaching 1392 °C, ferrite is formed.
Ferrite
The carbon content will be 0.1%. Its atoms are located in defective lattice sites.
Ferrite is close in its parameters to iron.
Hypoeutectoid steel: structure, properties, production and application
The use of carbon steels is widespread in construction and industry. The group of so-called technical iron has many advantages that lead to increased performance qualities of final products and structures. Along with optimal characteristics of strength and load resistance, such alloys are also distinguished by flexible dynamic properties. In particular, hypoeutectoid steel, which also contains a considerable percentage of carbon mixtures, is valued for its high ductility. But this is not all the advantages of this type of high-strength iron.
General information about the alloy
A distinctive property of steel is the presence of special alloyed impurities and carbon in the structure. Actually, a hypoeutectoid alloy is determined by its carbon content. Here it is important to distinguish between classical eutectoid and ledeburite steels, which have much in common with the described type of industrial iron. If we consider the structural class of steel, then the hypoeutectoid alloy will be classified as eutectoids, but containing alloyed ferrites and pearlites. The fundamental difference from hypereutectoids is the level of carbon, which is below 0.8%. Exceeding this indicator allows steel to be classified as a full-fledged eutectoid. In some ways, the opposite of hypoeutectoid is hypereutectoid steel, which, in addition to pearlite, also contains secondary carbide impurities. Thus, there are two main factors that make it possible to distinguish hypoeutectoid alloys from the general group of eutectoids. Firstly, it is a relatively low carbon content, and secondly, it is a special set of impurities, the basis of which is ferrite.
Reading an Iron-Carbon Diagram
We can see the composition of an alloy with a given initial carbon content at a given temperature by moving along a vertical line corresponding to the carbon content in the alloy.
Consider, for example, the AEC region. It is adjacent to areas of austenite AESG and the liquid phase. The alloys in it consist of a liquid phase and the resulting solid austenite. How to determine the carbon concentration in different phases for a given alloy? Let us consider, as an example, an alloy with an initial carbon concentration of 2.5% at a temperature of 1250°C.
Let’s draw a horizontal straight line from this point on the graph “2.5% C – 1250°C”. The intersection of this straight line with the AE line bordering the austenite region will show the carbon concentration in austenite at a given temperature (~1.5%).
The intersection of the same horizontal line with line AC, bordering the liquid phase region, will show the concentration of carbon in the liquid phase at a given temperature (~3.5%).
This is how we can determine the carbon concentration in the phases of any alloy at a given temperature:
- in the liquid phase and austenite in the AEC region;
- in the liquid phase in the CDF region (carbon concentration in cementite, of course, is constant - 6.67%);
- in austenite in the SEFK region;
- in ferrite in the QPKL region;
- in ferrite and austenite in the GPS region.
As we can see, at a carbon concentration above 2.14%, the saturation of the cooled melt with carbon always tends to 4.3% (along the AC and DC lines) as it approaches a temperature of 1147°C (ECF level). Next, the liquid transforms into ledeburite (eutectic). Naturally, with the same average carbon content.
As the temperature approaches 727°C (PSK level), the carbon concentration in austenite (“free” and/or included in ledeburite) tends to 0.8% (according to the GS and ES lines). Next, the transformation of austenite into pearlite (eutectoid) occurs. Perlite, of course, has an average carbon content of 0.8%.
Manufacturing technology
The general technological process for producing hypoeutectoid steel is similar to the production of other alloys. That is, approximately the same technical techniques are used, but in different configurations. Hypoeutectoid steel requires special attention in terms of obtaining its specific structure. For this purpose, technology is used to ensure the decomposition of austenite against the background of cooling. In turn, austenite is a combined mixture, including the same ferrite and pearlite. By regulating the intensity of heating and cooling, technologists can control the dispersion of a given additive, which ultimately affects the formation of certain performance qualities of the material.
However, the carbon value provided by perlite remains at the same level. Although subsequent annealing may alter the formation of the microstructure, the carbon content will be within 0.8%. Normalization is an obligatory stage in the process of steel structure formation. This procedure is required for fractional optimization of grains of the same austenite. In other words, ferrite and pearlite particles are reduced to optimal sizes, which further improves the technical and physical characteristics of steel. This is a complex process in which much depends on the quality of heating control. If the temperature regime is exceeded, then the opposite effect may well be achieved - an increase in austenite grains.
Annealing steel
Several annealing methods are used. The techniques of complete and incomplete annealing are fundamentally different. In the first case, austenite is intensively heated to a critical temperature, after which normalization occurs through cooling. Austenite decomposes immediately. As a rule, complete annealing of steels is carried out at 700-800 °C. Heat treatment at this level just activates the processes of decomposition of ferrite elements. The cooling speed can also be adjusted, for example, the oven operator can control the chamber door by closing or opening it. The latest models of isothermal furnaces can automatically perform slow cooling in accordance with a given program.
As for incomplete annealing, it is performed by heating at temperatures above 800 °C. However, there are serious limitations on the retention time of the critical temperature effect. For this reason, incomplete annealing occurs, as a result of which the ferrite does not disappear. Consequently, many shortcomings in the structure of the future material are not eliminated. Why is such annealing of steels necessary if it does not improve physical properties? In fact, it is incomplete heat treatment that allows the soft structure to be preserved. The final material may not be required in every application typical of carbon steels per se, but will allow easy machining. A soft hypoeutectoid alloy can be cut without much difficulty and is cheaper to manufacture.
Alloy normalization
After firing comes the turn of increased heat treatment procedures. There are normalization and heating operations. In both cases we are talking about a thermal effect on the workpiece, at which the temperature can exceed 1000 °C. But the normalization of hypoeutectoid steels itself occurs after the completion of heat treatment. At this stage, cooling begins under calm air conditions, during which exposure occurs until the complete formation of fine-grained austenite. That is, heating is a kind of preparatory operation before bringing the alloy to a normalized state. If we talk about specific structural changes, then most often they are expressed in a decrease in the size of ferrite and pearlite, as well as in an increase in their hardness. The strength properties of particles are increased in comparison with similar characteristics achieved by annealing procedures.
After normalization, another heating procedure with a long exposure may follow. The workpiece is then cooled, and this step can be performed in different ways. The final hypoeutectoid steel is produced either in air or in a slow-cooling furnace. As practice shows, the highest quality alloy is formed using complete normalization technology.
Effect of temperature on the structure of the alloy
The intervention of temperature in the process of formation of the steel structure begins from the moment of transformation of the ferrite-cementite mass into austenite. In other words, perlite becomes a functional mixture, which partly becomes the basis for the formation of high-strength steel. In the next stage of thermal treatment, the hardened steel gets rid of excess ferrite. As already noted, it is not always completely eliminated, as in the case of incomplete annealing. But the classic hypoeutectoid alloy still involves the elimination of this austenite component. At the next stage, the existing composition is optimized with the expectation of forming an optimized structure. That is, the alloy particles decrease with the acquisition of increased strength properties.
Isothermal transformation with a supercooled mixture of austenites can be performed in different modes, and the temperature level is only one of the parameters controlled by the technologist. Peak intervals of thermal exposure, cooling rates, etc. also vary. Depending on the selected normalization mode, hardened steel with certain technical and physical characteristics is obtained. It is at this stage that it is also possible to set special operational properties. A striking example is an alloy with a soft structure, obtained for the purpose of efficient further processing. But most often, manufacturers still focus on the needs of the end consumer and his requirements for the basic technical and operational qualities of the metal.
Steel structure
The normalization mode at a temperature of 700 °C causes the formation of a structure in which the basis will be ferrite and pearlite grains. By the way, hypereutectoid steels have cementite in their structure instead of ferrite. At room temperature, in the normal state, there is also a content of excess ferrite, although as carbon increases, this part is minimized. It is important to emphasize that the structure of steel depends to a small extent on the carbon content. It has virtually no effect on the behavior of the main components during the same heating process and is almost entirely concentrated in perlite. Actually, perlite can be used to determine the level of carbon mixture content - as a rule, this is an insignificant value.
Another structural nuance is also interesting. The fact is that pearlite and ferrite particles have the same specific gravity. This means that by the amount of one of these components in the total mass, you can find out what the total area it occupies is. In this way, microsection surfaces are studied. Depending on the mode in which the hypoeutectoid steel was heated, the fractional parameters of austenite particles are also formed. But this happens almost in an individual format with the formation of unique values - another thing is that the limits for various indicators remain standard.
Components, phases and structural components of iron-carbon alloys
Iron
– ductile metal of silver-white color. The hardness and strength of iron is low ( HB80
,
σв = 250 MPa)
with significant plasticity
(δ=50%).
Melting point
– 1539º C
, density
7.83 g/cm2
.
Carbon
found in nature in the form of diamond and graphite.
Graphite has a complex crystal lattice.
It is a fragile material, but with increasing temperature, the strength of graphite increases significantly. The melting point of graphite
is
3500º C. With carbon, iron forms chemical compounds
and
interstitial solid solutions.
The chemical compound of iron and carbon (iron carbide)
Fe3C
is called cementite.
It contains
6.67%
carbon (by mass).
Cementite has a complex rhombic crystal lattice, is characterized by very high hardness ( HB800
), brittleness and extremely low ductility.
The melting point depends on its composition (chromium, molybdenum, etc.) and is in a wide range of 1250…1600º C.
A solid solution of carbon in α-iron is
called ferrite.
The carbon content in ferrite is very low - maximum - 0.02%
at a temperature of
727ºС (Fig. 2.2)
.
At room temperature, ferrite contains no more than 0.006%
.
Due to such a low carbon content, the properties of ferrite coincide with the properties of iron (low hardness and high ductility). A solid solution of carbon in δ-iron, existing at a temperature of 1392…1539º C
, is also called
ferrite,
or
high-temperature ferrite
(
δ-ferrite
).
It is characterized by a maximum carbon solubility of 0.1%
at a temperature of
1499ºC.
A solid solution of carbon in γ-iron is
called austenite
. The maximum carbon content in austenite is 2.14%
(at a temperature of
1147ºС
).
Austenite is characterized by high ductility and low strength and hardness ( HB220
).
A mechanical mixture of ferrite and cementite is called pearlite.
He contains 0,8%
carbon and is formed from austenite at a temperature of
727º C
and is
a eutectoid
.
A eutectoid
is a mechanical mixture of two phases formed from a solid solution
.
Perlite has a lamellar structure, i.e. consists of alternating plates of ferrite and cementite. A granular structure of pearlite is also possible, when it consists of cementite grains surrounded by ferrite. Granular perlite is much more plastic than lamellar pearlite and has lower hardness.
A eutectic mixture of austenite and cementite is ledeburite.
He contains 4,3%
carbon, is formed from a liquid alloy at a temperature of
1147º
C. At a temperature of 727º C,
austenite, which is part of ledeburite, turns into pearlite, and below this temperature ledeburite is a mechanical mixture of pearlite and cementite.
Ledeburite has high hardness (HB600...700)
and brittleness.
The cementite phase has five structural forms: primary cementite
, formed from a liquid alloy; secondary cementite
, formed from austenite;
tertiary cementite
, formed from ferrite;
ledeburite cementite
;
cementite perlite. Iron-cementite diagram
Iron-carbon alloys containing up to 6.67%
(steel and cast iron). Therefore, the phase diagram of iron-carbon alloys is considered only up to this concentration, i.e. in fact, the iron-cementite diagram ( Fe–Fe3C)
).
Figure 2.2
shows a diagram of the state of iron alloys with cementite.
The horizontal concentration axis shows carbon content from up to 6.67%.
The left vertical axis corresponds to
100%
iron content.
It shows the melting point of iron and the temperature of its polymorphic transformations. The right vertical axis ( 6.67%
carbon) corresponds to
100%
cementite content. The letter designation of the points on the diagram is adopted in accordance with the international standard and is not subject to change.
Properties of hypoeutectoid steel
This metal belongs to low-carbon steels, so you shouldn’t expect any special performance qualities from it. Suffice it to say that in terms of strength characteristics, this alloy is significantly inferior to eutectoids. This is due precisely to differences in structure. The fact is that the hypoeutectoid class of steel containing excess ferrites is inferior in strength to analogues that have cementite in their structural composition. Partly for this reason, technologists recommend for the construction industry the use of alloys in the production of which the firing operation with the displacement of ferrites was maximally implemented.
If we talk about the positive exceptional properties of this material, then they consist of ductility, resistance to natural biological processes of destruction, etc. At the same time, hardening of hypoeutectoid steels can add a number of additional qualities to the metal. For example, this may include increased thermal resistance, lack of susceptibility to corrosion processes, as well as a whole set of protective properties inherent in conventional low-carbon alloys.
Nodal critical points of the iron-carbon system state diagram
On the iron-carbon diagram there are a number of points called critical points. Each point carries information about temperature, carbon content and a description of what exactly is happening in that place.
There are 14 of these critical points in total.
For example, A, says that at a temperature of 1539 ° C and at zero carbon content, pure iron melts. D indicates that at a temperature of 1260 Fe3c can melt.
The points are located at the intersection of lines placed on the diagram.
Areas of application
Despite a slight decrease in strength properties due to the metal belonging to the class of ferritic steels, this material is widespread in different areas. For example, in mechanical engineering, parts made of hypoeutectoid steels are used. Another thing is that high grades of alloys are used, in the manufacture of which advanced firing and normalization technologies were used. Also, the structure of hypoeutectoid steel with a reduced ferrite content makes it possible to use the metal in the production of building structures. Moreover, the affordable cost of some grades of steel of this type allows you to count on significant savings. Sometimes in the manufacture of building materials and steel modules, increased strength is not required at all, but wear resistance and elasticity are required. In such cases, the use of hypoeutectoid alloys is justified.
Austenite in steels
The presence of austenite in steel alloys gives them certain properties. Parts and assemblies made from such steels are intended to work in environments containing aggressive components, for example, in enterprises processing various acids.
Steels of this class are characterized by a high level of alloying; during crystallization, a face-centered lattice is formed. This structure is not subject to change even under the influence of deep cold.
Steels of this type can be divided into two types that differ from each other in composition. Firstly, they contain substances such as iron, nickel, chromium. In this case, the total number of additives cannot exceed 55%. The second group includes nickel and iron-nickel compositions. In nickel compositions, its content exceeds 55%. In iron-nickel compositions, the ratio of nickel to iron is 1:5, and the amount of nickel starts from 65%.
This amount of nickel provides increased ductility, and chromium, in turn, provides high corrosion resistance and heat resistance. The use of other alloying materials makes it possible to smelt alloys with unique performance properties. Metallurgists, when formulating alloys, are guided by the future purpose of the steels.
To produce alloy steels, ferritizers are used, which impart constancy to austenites; such substances include niobium, silicon and some others. In addition to them, carbon and manganese are used - they are called austenizers.
Production
Many enterprises in Russia are engaged in the production, preparation and production of hypoeutectoid metal. For example, the Ural Non-Ferrous Metals Plant (UZTM) produces several grades of steel of this type, offering the consumer different sets of technical and physical properties. The Ural Steelworks produces ferritic steels, which contain high-quality alloy components. In addition, the range includes special modifications of alloys, including heat-resistant, high-chromium and stainless steels.
Among the largest manufacturers, one can highlight the Metalloinvest enterprise. At the facilities of this company, structural steels with a hypoeutectoid structure are produced, designed for use in construction. At the moment, the company's steel mill operates according to new standards, which make it possible to improve the weak point of ferrite alloys - the strength indicator. In particular, the company's technologists are working on increasing the stress intensity factor, optimizing the impact strength and fatigue resistance of the material. This allows us to offer alloys for almost universal purposes.