BrB2 is a tin-free beryllium bronze that is processed by pressure. The chemical composition of the BrB2 alloy is described in GOST 18175-78 and includes the following components: copper 96.9-98.0%, beryllium 1.8-2.1%, nickel 0.2-0.5% and up to 0. 5% impurities. The alloy stands out among other bronzes due to its high wear resistance and resistance to corrosion fatigue. Along with other bronzes, BrB2 has good antifriction and springing properties, as well as average heat and electrical conductivity, which determines the use of BrB2 tape and wire. In addition, the mechanical properties of this alloy can be improved by subjecting it to hardening and aging procedures. For example, BrB2T rod is widely used.
Properties of BrB2
Let's consider the properties of beryllium bronze grade BrB2 - chemical, technological, mechanical, physical.
Chemical composition of BrB2
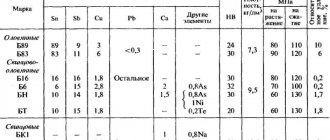
Chemical composition of BrB2 alloy according to GOST 18175 - 78
| Fe | Si | Ni | Al | Cu | Pb | Be | Impurities |
| up to 0.15 | up to 0.15 | 0.2 — 0.5 | up to 0.15 | 96.9 — 98 | up to 0.005 | 1.8 — 2.1 | only 0.5 |
Note: Cu
- the basis;
the percentage of Cu
is given approximately.
Foundry and technological properties of BrB2 bronze
| Melting point of BrB2 | 955 °C |
| Hot processing temperature BrB2: | 750 - 800 °C |
| Annealing temperature BrB2: | 530 – 650 °C |
Mechanical properties of BrB2
| Assortment | Short-term strength limit sв | Proportional limit (yield strength for permanent deformation) sT | Elongation at break d5 |
| — | MPa | MPa | % |
| Soft wire, GOST 15834 - 77 | 343-686 | 15-60 | |
| Hard wire, GOST 15834 - 77 | 735-1372 | ||
| Soft strip, GOST1789-70 | 390-590 | 20-30 | |
| Hard strip, GOST 1789-70 | 590-930 | 2.5 | |
| Soft alloy, GOST1789-70 | 400-600 | 196-344 | 40-50 |
| Hard alloy, GOST1789-70 | 600-950 | 588-930 | 2-4 |
The hardness of BrB2 rods is specified in GOST 15835-2013 (replacing GOST 15835-70)
Hardness BrB2
| Hardness BrB2, soft rod GOST 15835-2013 | HB 10 -1= 100 – 150 MPa |
| Hardness BrB2, Hard rod GOST 15835-2013 | HB 10 -1= 150 MPa |
HB - Brinell hardness of beryllium bronze
Physical properties of BrB2 (beryllium bronze)
| Temperature T | Modulus of elasticity of the first kind E 10-5 | Coefficient of thermal (linear) expansion a10 6 | Heat capacity l | Density | Specific heat capacity C | Electrical resistivity R 109 |
| hail | MPa | 1/Grad | W/(m deg) | kg/m3 | J/(kg deg) | Ohm m |
| 20 | 1.31 | 84 | 8200 | 70 | ||
| 100 | 16.6 | 419 |
Analogues of BrB2
| USA | Germany | Japan | |
| DIN,WNr | JIS | ||
Marking
All categories of bronzes are marked with Russian letters. The marking order is as follows:
1. Write the letters Br first.
2. Then letters denoting alloying elements:
3. Then write numbers indicating what percentage of each element is contained in the material.
But then the differences begin for deformable and casting grades:
- For deformable ones, the numbers are written at the end, in the same order as the corresponding alloying elements. Separated by hyphens. For example: BrAZh9-4. Tin free, pressure treated. Composition: aluminum - approximately 9%, iron - approximately 4%, the rest is copper.
- For foundries, the amount of the alloying element is written immediately after the corresponding letter. For example: BrO4Ts7S5. Foundry tin bronze. Composition: tin - approximately 4%, zinc - approximately 7%, lead - approximately 5%. The rest is copper.
There is no need to think that if only three elements are indicated in the marking, then there is nothing in the material except them and copper. It may also contain other substances. It's just that their number is very small. The chemical composition for different brands will be given below.
And further. Sometimes the letter L is placed at the end of the marking. This means that the alloy is cast, but with the same composition it can also be deformable.
Application of beryllium bronze BrB2
Rods made of BrB2 bronze are used in instrument making and automotive industry. BrB2 tapes are also used in instrument making and the production of elastic and spring parts. Wire has found a similar application in mechanical engineering and instrument making. Bronze BrB2 is used in various areas of production. Antifriction parts and spring parts are made from it: spring parts and springs. Critical parts are made from it. Non-sparking tools are also made from it.
Technological characteristics make it possible to produce complex high-quality castings from beryllium bronzes, but usually parts from them are made from blanks subjected to preliminary plastic deformation (sheets and strips, wire, tapes, etc.). The widespread use of beryllium group alloys is also due to the fact that they lend themselves well to various types of processing, and all known methods (welding and soldering) can be used to connect parts made from them.
Soldering and welding BrB2
Soldering of beryllium bronzes should be performed immediately after thorough mechanical cleaning of the elements to be joined has been performed. When performing such a technological operation, silver-based alloys are used as solder, and the protective flux, the use of which is necessary, must necessarily contain fluoride salts. High quality soldering of parts made of these alloys is ensured by a technology that involves performing the connection in a vacuum and using a layer of protective flux.
Parts made of beryllium bronzes are not joined using electric arc welding; other technologies are successfully used for this: spot, seam, roller and inert gas welding. This limitation in the use of electric arc welding is due to the fact that the alloys of this group have a large crystallization temperature range. In addition, welding of beryllium group bronzes cannot be performed after heat treatment, which is due to their special mechanical properties.
Wear resistance and corrosion resistance of BrB2 bronze
Parts made of beryllium bronze do not wear out and at the same time have a gentle effect on the mating mechanisms, fit well with each other, are polished and interact perfectly in the mechanisms under given parameters. But even if operating conditions are violated, parts made of BrB2 are able to withstand heavy friction loads and other mechanical influences. During the operation of mechanisms during wear, BrB2 does not break off in large pieces, but wears out gradually, producing very small chips.
Corrosion fatigue is one of the indicators of the corrosion resistance of metals. When parts operate under the influence of large mass, cyclic dynamic loads in a corrosive environment, there is a high probability of failure of the structures in which they are used. The BrB2 alloy performs well in various corrosive environments and can be used for the manufacture of critical parts, since corrosion manifests itself quite slowly and does not have a significant effect on the mechanical and physical properties of parts made from this material for a long time. However, under the influence of moist ammonia vapor and air, beryllium bronzes are prone to intercrystallization corrosion and cracking. In a gas environment saturated with halogens (fluorine, bromine, chlorine and iodine), beryllium halides form on their surface, which causes a decrease in its concentration in the alloy. The process of interaction with halogens occurs especially actively at elevated temperatures. In this regard, beryllium bronze BrB2 is not recommended for use in the manufacture of parts used in these gases.
What industries use copper-beryllium alloys?
The described bronzes, which have unique properties, are used in those industrial areas where increased demands are placed on parts made from them. The production process of copper-beryllium compositions is quite expensive, so they are used only in “special” cases.
They are most actively used in electronic and electrical products:
- in telecommunications fiber optic technology;
- in various connectors, spring contacts;
- in socket-type connectors for creating integrated circuits.
Also, nowadays not a single portable electronic device can do without beryllium compositions, be it a laptop, tablet computer, cell phone or communicator. From copper and beryllium alloys it is possible to produce miniature parts, which are exactly what is required for these devices.
Photo of an alloy of copper and beryllium
The bronzes in question are also used in the manufacture of equipment for oil production, as well as drilling rigs. Corrosion resistance, high anti-friction and strength – these are the properties of Cu–Be systems that are of interest to drillers and oil workers. Typically, auxiliary drilling devices, drill pipes and threaded connections for them, and pump supports for oil pumping are produced from copper-beryllium alloys.
Other applications of copper and beryllium based alloys:
- Automotive industry. Nowadays, the level of computerization of vehicles is constantly increasing. And here it is difficult to do without miniature and at the same time extremely reliable parts that are made from beryllium-containing compositions. In any vehicle they are present in the form of components of electronic circuits of various automotive systems and elements of modern engines.
- Mechanical and aircraft manufacturing. Bronze in these industries is indispensable for structures that are operated under conditions of variable temperatures and loads. These include aircraft landing gear elements, aircraft navigation instruments, and critical components of machines and mechanisms.
- Contact welding. The high electrical conductivity and heat resistance of beryllium-containing alloys (such properties are especially characteristic of low-alloy bronzes) determine their demand in the production of electrical holders and welding rods, which are characterized by a long service life. Such electrodes are recommended for connecting railway rails, steel sheets, and any types of reinforcement and wire.
In the photo - beryllium-containing alloy
Another area of application of bronzes with beryllium is the manufacture of pistons of units that are used to perform casting operations under pressure, walls of equipment for crystallization of continuous casting machines and injection molding equipment, molds for casting all kinds of complex alloys and metals. In this case, there is no need for additional protection of the walls of these units in order to increase their operating time.
Upgrading and hardening of BrB2
By refining, products made from BrB2 are harder and more ductile. Accordingly, semi-finished products are produced in soft (M) and hard (T) states. During the hardening procedure, the metal is heated to a certain temperature, after which it is cooled in water. As a result, the plastic properties of the metal increase and it is used for the manufacture of parts by rolling, forging, drawing and cold bending. Semi-finished products from BrB2 with hardening and cold deformation are also produced. BrB2 is hardened at a temperature of 750-790 °C, after which the alloy is tempered at a temperature in the range of 300-350 °C. After cold deformation, the mechanical properties of hardness, strength and fluidity are improved. BrB2 T stands out among other bronzes for its highest tensile strength. Copper-beryllium alloy BrB2, subjected to thermal hardening, becomes more durable, elastic and ductile. Initially, it is brought into a soft state by heating to 760-780°C, and then subjected to aging in water at a temperature of 310-330°C for 3 hours. When the alloy is heated and then cooled to room temperature, beryllium dissolves in copper to form a saturated solid solution. Subsequent hardening leads to its precipitation, as a result of which BrB2 bronze acquires high hardness up to 350 - 400 HB.
Beryllium copper alloy - what is it?
Beryllium bronze is a dispersion-hardening alloy of the copper-beryllium (Cu-Be) system with a beryllium content of 1.6 to 3 percent. Also considered such bronzes are the “copper-beryllium-cobalt” (abbreviated as IBC) and “copper-beryllium-nickel” (MNB) systems. MKB and MNB may contain no more than 0.8 percent beryllium.
The peculiarity of beryllium-containing bronzes is that with a change in temperature, the solubility of the alloying elements present in them also changes. In a solid solution during quenching from a single-phase zone, the formation of an increased number of alloying additive atoms is observed (if we compare their number at the equilibrium state of a particular system). The resulting solid supersaturated solution from the point of view of thermodynamics is unstable.
At the slightest change in conditions, it disintegrates. As the temperature increases, the decomposition process becomes more intense, and as the temperature decreases, it slows down. The strengthening effect depends on the degree of dispersion of the precipitates that are formed during the decomposition of the specified solution.
Use in instrument making
It is the most common. Beryllium bronze is used in the production of electronic and electrical parts. First of all, these are spring contacts, connectors, and switches.
Also, the production of fiber optic telecommunications equipment cannot do without it. This category includes:
- Optical transmitters.
- Preamplifiers.
- Synchronization and data recovery chips.
- Blocks for converting serial code into parallel.
- Cables.
- Parallel-serial converters.
- Laser shapers.
Beryllium bronze is also used to produce socket connectors for connecting integrated systems to a printed circuit board.
And, as computer and mobile equipment becomes more complex with the development of technology, electronic parts are increasingly miniaturized. Demand for bronze-beryllium alloys is increasing, since they are the ideal material for the manufacture of small, lightweight and reliable parts. They are already used in many modern smartphones, communicators, laptops and tablets.
And computer connectors, switches, springs and other parts have been made from them for a long time. In 1999, it was estimated that each PC contained at least 2 grams of beryllium in bronzes.
Aircraft industry
Surely everyone is familiar with aircraft such as the Boeing 787 and Airbus A380. Beryllium alloy was used in the production of their cases.
The fact is that this is an ideal material for these purposes. It has much higher hardness, strength, wear resistance and load-bearing capacity than any other alloy. It forms a persistent but thin layer of oxide on the surface of the housing, which acts as a self-healing lubricant.
Bushings that experience repeated contact with the surface are also made from this alloy. It must remain smooth at all times, which means the material needs to be one that does not wear out. It is logical why beryllium alloy is used.
Plus, it has excellent thermal properties - thermal stability and a low coefficient of thermal expansion. These are the most important qualities that are taken into account in the first place in aerospace design. Alloys must withstand extreme temperatures.
By the way, another advantage of the material is its good fluidity in the molten state. This property makes it suitable for complex castings. Pitot tube bodies, for example, include extremely thin cast structures. And they can only be cast from a well-flowing material. And the guide blades of helicopter turbines, by the way, are also made from these alloys.
Main characteristics
The widespread use of this material is determined by its basic characteristics. Bronze is characterized by such distinctive features as:
- high corrosion resistance;
- strength;
- high level of electrical and thermal conductivity;
- increased wear resistance;
- low coefficient of friction process;
- excellent resistance to seawater, outdoors and various organic solutions;
- high steam resistance;
- ease of processing.
Bronze, whose melting point is about 930-1100 degrees, has excellent strength and durability. Especially when you compare it with other similar alloys.
Toxicity
In solid form and as finished products, beryllium copper poses no known health hazard.[ citation needed
] However, inhaling dust, mists, or fumes containing beryllium can cause serious lung disease. chronic beryllium disease. This disease primarily affects the lungs, limiting the exchange of oxygen between the lungs and the bloodstream. The International Agency for Research on Cancer (IARC) classifies beryllium as a Group 1 human carcinogen. The National Toxicology Program (NTP) also lists beryllium as a carcinogen.
References
- Bauccio, Michael (ed.). ASM Handbook of Metals, Third Edition
. Materials Park, Ohio: ASM International. p. 445. ISBN 0-87170-478-1 .CS1 maint: additional text: list of authors (link to site) - "C17200 Berryllium Copper." Aviva Metals
. - "Federal Law and Ammunition." Nucnews.net. Archived from the original on 2009-11-14. Retrieved 2009-11-02.
- "Archival copy." In the archive from the original dated June 27, 2009. Retrieved 2009-05-08.CS1 maint: archived copy as title (site link)
- "Feature - EDMing Beryllium Copper: Introduction." mmsonline.com. Archived from the original 2011-06-14. Retrieved 2010-10-17.
contact welding
This is a process during which a permanent welded joint is formed by heating the metal with a current passing through it, with further plastic deformation of this zone under the influence of compressive force. That is, also the technical sphere. Here, those made of beryllium alloy are used:
- Electrodes. Current conductors.
- Electrode holders. The part of the electric welding machine into which the electrode is inserted.
Their service life is much longer than that of those components that are made from other alloys (chrome bronze, to be more precise). The range of applications is wide. Here's what's welded using beryllium bronze components: wire, structural rebar, carbon steel sheets, nickel-chromium-silicon alloys, mainline rails, etc.
This is a truly wear-resistant material. Components made from it can work with high electrode pressing forces, powerful current and high speed. They can withstand a large number of welds because they have an increased modulus of elasticity, resistance to alternating loads and high softening temperatures. This means that not only the electrodes have to be changed less frequently, but the accuracy and strength of the connections increases several times.
Biological effects of beryllium
As an element, Be is present in the tissues of most flora and fauna. Thus, the concentration of beryllium in soil is thousandths of a percent; for plant ash this value is an order of magnitude less. Regarding animals, Be is evenly distributed throughout their tissues, with a total concentration ranging from ten thousandths to thousandths of a percent. When the body functions normally, half of the beryllium is excreted in the urine. The remainder of the element is deposited in the bones - 30%, liver and kidneys - 8% each.
Beryllium metal dust is very dangerous for humans
Is beryllium dangerous?
Volatile beryllium compounds and its dust are harmful to humans. As a result, processing Be requires compliance with certain safety standards, in particular the use of special protective measures to avoid beryllium poisoning.