Copper and bronze, also known as the “red metals,” may look similar at first glance, but they are actually completely different metals.
Copper and bronze are radically different in chemical composition, areas of use and properties. Copper is a pure chemical element and natural mineral that is found primarily in soil and, in smaller quantities, in water. In contrast, bronze is an alloy that contains copper as the main component, with tin and other metallic and non-metallic compounds. There are bronze alloys of various types, with different compositions; so different alloys have different properties and applications. Copper is an excellent electrical and thermal conductor. This metal is used in various fields. However, the key difference between copper and bronze is that copper is a pure chemical element and a natural mineral, while bronze is a metal alloy.
What is copper?
The word "Copper" comes from the Latin word "cuprum". It is a chemical element with atomic number 29 and the letter symbol "Cu". Copper is a ductile metal with very high electrical and thermal conductivity. Copper, due to its excellent electrical and thermal conductivity, corrosion resistance and good strength, is used to produce a wide range of industrial products. For example, copper is widely used as a conductor of electricity and heat, a building material, and in the production of various metal alloys. Pipes and fittings are primarily manufactured using copper due to its corrosion resistance.
There are many types of copper, which can vary in the amount of impurities they contain. Oxygen-free grades of copper are used in products that require ductility and conductivity.
One of the most important properties of copper is its ability to fight bacteria. After extensive antimicrobial testing by the Environmental Protection Agency, 355 copper alloys, including many brass alloys, were found to kill more than 99.9% of bacteria within two hours of contact. It is worth noting that the normal tarnishing of copper over time does not reduce its antimicrobial effectiveness.
Copper was one of the earliest metals discovered by man. The Greeks and Romans turned it into tools or jewelry, and there is even historical evidence that copper was used to sterilize wounds and purify drinking water. Today it is most often found in electrical products, such as wiring, due to its ability to efficiently conduct electricity.
Use of chemical reagents
The use of any reagents is a fairly effective, but destructive way to determine the type of copper alloy . It is carried out in several stages:
- it is necessary to remove chips from the surface of the sample or separate a small piece of thickness;
- prepare a solution of nitric acid (HNO3) in a 1:1 ratio with water;
- place the prepared shavings in the reagent;
- Heat the container with the acid solution to boiling temperature and maintain it until the chips are completely dissolved.
- The presence of brass will leave the solution clear without changing color .
- Bronze produces a white precipitate as a result of the reaction of tin with acid.
What is bronze?
Bronze is a metal alloy that contains copper as the main component and about 12 percent tin. Some other metals and non-metals can also be included in its composition, depending on the requirements, to obtain the desired properties. The most commonly added metals are aluminum, manganese, zinc or nickel. Examples of other components are silicon, phosphorus or arsenic. The addition of various metallic and non-metallic compounds results in a wide range of bronze alloys with varying properties.
Composition of copper and bronze
Copper is naturally present in soil as a mineral at a concentration of 50 parts per million. The main source of copper is copper sulfide (CuFeS2), which is also known as chalcopyrite. But it exists in its pure form as a natural mineral, not combining with other elements; this is "native copper". There are 29 isotopes of copper, of which only two types (63Cu and 65Cu) are stable, while the other isotopes are radioactive.
Bronze is a metal alloy containing copper (Cu) as the main element, the second important element being tin (Sn). Their percentage varies depending on the properties required, but most often the alloy contains about 12% tin and 88% copper. Their percentage changes slightly with the addition of other metals and non-metallic compounds.
There are many many bronze alloys and they have different properties depending on their use.
Main types of alloys:
- Commercial bronze: copper (90%), zinc (10%);
- Architectural bronze: copper (57%), zinc (40%), lead (3%);
- Plastic bronze: contains significant amounts of lead (Pb) to improve ductility;
- Phosphor bronze (or tin bronze): copper, tin (0.5% to 1.0%), phosphorus (0.01% to 0.35%);
- Aluminum Bronze: copper, aluminum (6% - 12%), iron (6% -max), nickel (6% -max);
- Silicon bronze: copper, zinc (20%), silicon (6%).
How to sell scrap bronze profitably, price per 1 kg at collection points
Most people associate the word “bronze” with objects of art and interior design:
- monuments;
- busts;
- antique candelabra;
- antique figurines;
- bells.
But bronze alloy , which is based on copper, is used not only in the artistic industry, but also in industry.
Before you sell bronze scrap, you should familiarize yourself with the types of this alloy, find out where it is used and how it is assessed at collection points. With such information, it is possible to get the maximum benefit from the transaction.
Prices and conditions of admission
Bronze scrap is a low-melting material that does not require a complex technological process for processing. Scrap is accepted continuously; the cost depends on the type of bronze scrap and its chemical composition.
Tin bronze has the highest price, since tin is an expensive non-ferrous metal, the cost of copper is several times higher.
According to GOST, the category of bronze scrap includes 15 items . Each company defines its own criteria when evaluating metal waste, but they are all united by the desire to obtain this type of non-ferrous scrap metal by any means.
Main types when assessing bronze:
- lump scrap;
- scrap mix;
- shavings;
- bronze products (casting).
Since bronze is very similar to brass, the main component of which is also copper, the alloy is analyzed using a special spectrometer.
Also, the composition of the alloy is determined using chemical reagents .
Large volumes of waste are more expensive.
Art and interior items are priced individually.
If the weight of bronze is more than 100 kilograms, it is possible for a specialist to visit the waste storage site to conduct an assessment. For non-cash payments, 10% is added to the accrued amount.
Below is information about how much bronze costs; average prices for 1 kg of scrap are given in table form:
| Scrap | 0-50 kg | 50-100 kg | 100-500 kg | From 500 kg - 1t |
| Scrap bronze - piece | 115-204 rub. | 170-215 rub. | 175-215 rub. | 190-228 rub. |
| Scrap mix | 180-184 rub. | 180-215 rub. | 180-215 rub. | 210-215 rub. |
| Shavings | 65–100 rub. | 100-170 rub. | 100-170 rub. | 110-180 rub. |
| Art and interior items | Price is negotiable | Price is negotiable | Price is negotiable | Price is negotiable |
Lump scrap includes bronze items with permissible contamination of up to 3% , a piece weighing no more than 100 kg .
Usually this:
- industrial waste,
- parts of super-power cranes,
- turbines
The main requirement is a homogeneous alloy composition of the entire batch.
Scrap with contamination up to 10% belongs to grade 2 ; scrap fragments can be made of various alloys.
Wood chips , industrial waste, in which the contamination is no more than 2%, are rated no less highly.
They are excellent for recycling without prior fractionation.
Of particular importance in the assessment of mixed alloys is the purity of the metal being submitted .
This indicator for bronze is not specified in GOST; it is agreed upon between both parties: the receiver and the client.
Many people are interested in the question - what is more expensive: copper or bronze? Having analyzed the average prices for receiving non-ferrous metal, you can find out that individual components of the classic alloy are more expensive than bronze itself - copper from 260 to 300 rubles per kilogram, and tin is even more expensive. And the price for scrap bronze does not exceed 215 rubles per 1 kg.
You can hand over bronze scrap to any non-ferrous scrap metal collection point.
Due to the similarity of the metal to brass, inspectors who do not use spectral analysis may mislead you into thinking that the metal is brass. The cost of this metal is less. To prevent this from happening, it is important to know the characteristic differences between these alloys.
How to distinguish bronze from brass:
- The color of the bronze alloy is darker than that of brass and has a characteristic reddish tint.
- Scratches remain on brass products due to mechanical impact .
- The fracture of the products differs in structure and color. Brass has a light-colored and fine-grained fracture surface. Bronze has a dark-colored fracture with a coarse-grained texture.
- Brass is never used in shipbuilding, since sea water has an aggressive effect on it.
Brass and copper are even more similar. Read more about how to distinguish them in a separate article.
To make it more profitable to hand over bronze scrap, it is preferable to agree on the handing over of the alloy with an organization that collects metal waste legally.
The following companies have metal analyzers
- spectrometers;
- steeloscopes;
- laboratories for chemical analysis.
When an appraiser visits a client to inspect scrap, an express analysis of alloys is possible, which will affect the objective value of the metal.
If a sufficient amount of metal waste has accumulated, then a large company will undertake the delivery of scrap to one of its collection points.
for legal entities include:
- preparation of documents for disposal in compliance with all legal regulations;
- free dismantling of bulky structures;
- collection of recyclable materials using your own special transport.
But it is not always possible to find such a company everywhere and not always.
If you turn to private traders involved in collecting scrap metal, the price will be more modest, but there will be no transportation costs. Don't forget to ask them about their license.
In any case, the scrap metal will go to its intended destination, namely to a processing plant, where it will be used to create secondary metal for the needs of metallurgy and other industrial sectors.
Recycling scrap bronze is saving copper natural resources .
It is impossible to imagine the life of a modern person without the use of metals. Landfills of rusting metal trash not only pollute the environment, but also lead to the gradual depletion of natural resources.
This situation is especially true with copper-containing ores and minerals that are not replenished by nature.
Selling scrap bronze is beneficial for people and the planet .
If, after reading the article, you change your mind about handing over scrap bronze, then watch the video that will help you melt it down yourself:
Use of copper and bronze
Copper has a wide range of uses in many areas; mainly in electrical, roofing and plumbing applications due to its high electrical conductivity, corrosion resistance and durability. It is also used in architecture, in coinage, and in the manufacture of various alloys and machine parts. Small amounts of copper are used to produce food additives and fungicides.
Bronze is widely used in shipbuilding, in the manufacture of parts and mechanisms for boats. One of the advantages of bronze is its resistance to seawater corrosion. It is also used to produce medals and musical instruments.
Melting temperature
Almost every type of metal has a different melting point. It is therefore not surprising that copper and bronze differ in melting point.
Pure copper melts at about 2000 F (1090 C).
Bronze has a lower melting point than pure copper because bronze is often made by combining copper with tin, which has a melting point of 450 F (230 C). Thus, the melting point of bronze will fluctuate between these two values, up or down, depending on the percentage of copper and tin.
How to distinguish bronze from brass: composition, characteristics, features
It is no coincidence that the question of how to distinguish bronze from brass interests many, because products made from these copper alloys are very similar in appearance. Meanwhile, having decided to use products made from such materials for a specific purpose, one should distinguish between these two metals, since they have serious differences in many respects.
These busts are very similar, but they are made from different copper alloys
What are bronze and brass
Bronze and brass are alloys based on copper. Moreover, individual brands of such alloys are very similar in color, but their characteristics may have significant differences. In order to have a good understanding of the question of in which cases to use brass and in which to use bronze, it is necessary to become more familiar with their properties and chemical composition.
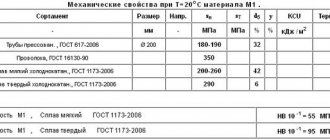
Chemical composition of simple brasses
Chemical composition of tin bronzes (click to enlarge)
A material such as bronze has been used by humanity for several millennia, and its popularity is not decreasing. Initially, man learned to produce bronze alloys, the basis of the chemical composition of which is copper and tin.
Later, with the development of the metallurgical industry, bronzes began to be produced, in which tin was replaced by other chemical elements - aluminum, lead, iron, silicon, beryllium, phosphorus, etc. Bronzes of the first type began to be called tin (they are often called bell bronzes, because they used to made bells), and the second - tinless.
A change in the chemical composition of bronze leads to a change not only in its characteristics, but also in color.
Brass is also a copper alloy, but its main alloying element is zinc. The chemical composition of various brands of brass may contain elements such as nickel, lead, iron, tin, manganese, etc.
, but their content is insignificant and is necessary only to give the finished alloy certain characteristics. It is known that the ancient Romans knew how to produce brass, who obtained it by mixing molten copper and zinc ore.
A more efficient production technology, which involves mixing molten copper and pure zinc, was developed in England, and this happened in 1781.
Physical properties of simple brasses (click to enlarge)
Physical properties of tin bronzes (click to enlarge)
For a long time, brass, which has a beautiful light golden color, was used to make decorative items, including those that were passed off as gold. However, manufacturers could not help but pay attention to other, no less significant characteristics of this alloy, which include high corrosion and abrasion resistance, ductility, combined with fairly high hardness and strength.
That is why brass, which also has good casting properties, began to be actively used not only for decorative purposes, but also for the manufacture of products successfully used in various industries.
Comparative characteristics
The basis of bronze and brass, as mentioned above, is the same metal - copper. The difference between these alloys lies in their chemical composition and, accordingly, in the characteristics that they possess. Naturally, the differences between these copper alloys also determine their areas of application.
Due to the fact that bronze is a stronger and more durable material when compared with brass, bells, sculptural compositions, elements of fencing, landscape and interior structures have been made from this material since ancient times.
It is also important that many grades of this alloy are characterized by good fluidity in the molten state. This makes it possible to cast products of even very complex configurations from them.
By adding various chemical elements to the chemical composition of bronze, you can change its color in a fairly wide range, which is also of great importance in the production of decorative products.
This watch ring, judging by the color, is more likely yellow brass (bronze would be redder). Scratches easily remain on the surface - also a sign of brass
Brass differs from bronze in its higher ductility and, accordingly, lower strength and wear resistance, which limits the use of this alloy in many areas. In addition, brass is less resistant to aggressive environments, in particular salty sea water, which does not allow the use of brass products in shipbuilding, where bronze is used very actively and successfully.
There is also a noticeable difference in the color of these alloys and in their internal structure. Any experienced specialist can tell you how to distinguish brass from bronze: to do this, just look at the fracture of products made from these alloys. Brass has a lighter color when fractured and a distinct fine-grained structure, while bronze is easily identified by its dark brown fracture color and coarse-grained internal structure.
Summarizing all of the above, we can highlight the following differences between brass and bronze.
- The main alloying element in bronze is tin, and in brass it is zinc. Moreover, both alloys are created on the basis of one metal – copper.
- Bronze (even with a classic chemical composition) perfectly resists the effects of aggressive environments, in particular salty sea water. In order to improve the corrosion resistance of brass, additional alloying elements must be introduced into such an alloy.
- The strength and anti-friction characteristics of bronze are also better than those of brass. Such qualities significantly expand the scope of application of bronze alloys, from which not only strong and durable decorative elements are made, but also critical parts for use in various industries. Brass is more often used for the production of bimetallic elements (“steel - brass”), which demonstrate high resistance to the formation and development of corrosion processes.
- Bronze products have a dark brown color and coarse grain when broken, while brass products have a yellow-golden color and a fine-grained structure. This difference in color and internal structure makes it easy to determine what alloy the product is made from.
- Bronze, like brass, although they are based on a metal such as copper, are divided into completely different categories. Thus, bronze can be tin or tin-free, while brass can be two- or multi-component.
Comparison of properties of brass and bronze
Bronze and brass, which have a lower melting point than copper, can be used to make various products at home. However, for this, naturally, it is necessary to stock up on the appropriate equipment and thoroughly study the technology and rules for performing such a technological operation as casting.