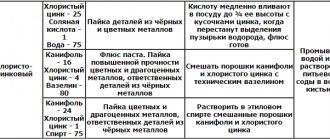
In the modern world, the procedure for treating metals with various substances that isolate them from the aggressive effects of working environments has become very popular.
Very often, galvanization is used for these purposes - an electrochemical method of applying a metal film that prevents oxidation and corrosion of surfaces, giving them hardness, wear resistance and an aesthetic appearance.
Galvanization promotes better thermal stability of metals, therefore this processing method is widely used in those industries that carry out technological operations at high temperatures.
In this article we will answer the most common questions related to electroplating:
- How did it come about?
- Where is it used?
- How and with what materials is it carried out?
- read about this below.
History of electroplating
Electroplating was discovered by Russian physicist Boris Jacobi in 1836. During his experiments, he passed metals through water and salt solutions under the influence of an electric current.
As they passed through salt solutions, the metals disintegrated into ions with different charges. Negative ones were deposited on the anode, positive ones - on the cathode (its role in galvanization is played by metals that need protection).
What is the essence of the galvanic process?
To understand what electroplating is, it is important to understand the essence of such an electrochemical process. Galvanic processing of a product, during which a thin metal layer is formed on its surface, can be divided into several main stages:
- preparation of an electrolytic solution, the composition of which is selected in each specific case;
- immersion in an electrolytic solution of two anodes connected to the positive contact of a direct current source;
- immersion in a solution for galvanizing the workpiece, placing it between the anodes and connecting it to the negative contact of an electric current source (thus, the workpiece will act as a cathode);
- closing the formed electrical circuit.
Galvanic bath diagram
Galvanic processes that begin to occur in such an electrical circuit consist in the fact that positively charged particles of the applied metal contained in the electrolyte solution, under the influence of electric current, begin to tend to the negatively charged cathode-product, settling on its surface and forming a thin metal film on it .
Galvanization principle
Before starting the process, metal surfaces are thoroughly cleaned of dirt and degreased.
Preliminary preparation of products is very important; the quality of galvanization depends on it.
For metal surfaces, there are special products based on organic solvents that do not cause metal corrosion.
For example, the MODENGY metal cleaner ensures the removal of contaminants of various chemical natures - petroleum products, silicone oils, preservative compounds, adsorbed gas films, moisture, etc.
In most cases, preparing a product for electroplating is not limited to just cleaning its surface and degreasing. Sandblasting and subsequent grinding using sandpaper and special pastes are also performed.
Galvanic coating highlights all the imperfections of the surface, so the part being processed must be perfectly prepared - without chips, scratches or cavities.
The scheme by which metal electroplating is implemented is quite simple.
The cleaned product is placed in a container with an electrolyte solution, and a negative charge is applied to it (the product becomes a cathode). A special metal plate, which will serve to form the coating, is charged positively and takes on the functions of an anode. When the electrical network is closed, the metal of the anode (plate) dissolves in the electrolyte and rushes to the negatively charged product (cathode), on which it creates a thin, uniform film.
This method of applying galvanic coatings is called anodic. Thanks to it, when a threat of corrosion arises, the galvanic insulation is destroyed, while the metal remains intact for a long time.
With cathodic deposition, which is used much less frequently, the slightest violation of the integrity of the applied layer leads to even more intense destruction of the metal underneath, which is facilitated by the coating technology itself.
An electrolyte is a conductive solution for moving metals from the anode to the cathode. The size of containers with this substance varies and depends on production tasks.
Larger items are held in large canopy baths. Smaller parts are electroplated in drum-type containers in which a negative charge is applied to a drum rotating in an electrolyte. For processing very small products (for example, hardware or other fasteners), there are bell-type filling baths: during operation they rotate slowly, as a result of which the parts are evenly coated with protective metal.
The current density passing through the electrolyte is of great importance, as it affects the structure of the formed deposit. This value is measured as the ratio of current per unit surface of the workpiece.
If the density is low, no sediment is formed at all; if the density is too high, there are a lot of powder deposits, which negatively affects the quality of the coating. That is why the galvanization process requires constant monitoring.
The thickness of the galvanic coating on finished products usually ranges from 6 to 20 microns and is determined by the characteristics of the materials involved in galvanization. The level of adhesion of the metal alloy to surfaces is determined by special tests.
Equipment and materials
Electroplating of various metals requires the use of appropriate equipment and consumables. For chrome plating, galvanizing, as well as for coating workpieces with other metals, the same type of galvanic equipment is used. The differences when performing such processes will only be in the composition of the electrolyte used, its temperature and other processing modes.
Metal processing by galvanizing is performed using equipment such as:
- galvanic baths into which an electrolytic solution is poured, anodes and the workpiece are placed;
- DC source equipped with an output voltage regulator;
- a heating device with which the electrolytic solution is brought to the required operating temperature.
Galvanic bath with rocking mechanism
Electroplating also requires anode plates, which can be made from a variety of metals. The purpose of such plates is not only to supply electric current to the electrolyte, as well as to uniformly distribute the current over the surface of the workpiece, but also to replenish the loss of metal deposited on the part, which is actively consumed from the electrolyte.
Different types of electroplating are applied using electrolytic solutions with different chemical compositions. To prepare such solutions, hazardous chemicals are used, so they must be stored in sealed glass containers with ground-in lids. All chemical reagents from which the electrolytic solution is prepared for electroplating must be measured in precise quantities, so to perform this procedure it is necessary to use electronic scales.
Manual electroplating line for precious metals
Any line for metal electroplating or simple electroplating equipment must be installed in rooms equipped with an effective ventilation system. It is also necessary to take very seriously the personal safety of the specialist servicing the electroplating equipment. All work related to electroplating must be performed in a respirator and safety glasses, thick rubber gloves, an oilcloth apron and shoes that can protect the skin of the feet from burns. If this process is performed at home, and you still do not fully know what galvanization is, then you should carefully study special literature in advance or watch a training video on this topic.
Are all metals compatible with each other?
When carrying out the electroplating process, there is the concept of material compatibility. All metals in joints corrode. In some cases, this process is slow. However, there are pairs that cannot be combined together.
Working with aluminum and its alloys is accompanied by certain difficulties, since there is an oxide film on their surfaces, which complicates the galvanization process.
When galvanizing aluminum, the following combinations include: copper – nickel – chromium; nickel – chromium; lead - tin; copper - tin. Brass plating and galvanizing of aluminum are allowed.
Types of galvanic coatings
Depending on the purpose of using finished products, galvanic coatings are divided into protective, protective-decorative and special.
Protective ones isolate metal parts from exposure to aggressive environments and protect them from mechanical damage.
Protective and decorative ones prevent the destruction of products under the influence of external factors, and also give them an aesthetic appearance.
Galvanic coatings for special purposes are applied to give parts new and improved characteristics: increased wear resistance and hardness, magnetic, electrical insulating properties.
In some cases, galvanization is used to restore the original appearance of a product after long-term use.
Electroplating allows you to create exact copies of parts that have even very complex reliefs. This process is called electroplating.
Depending on the materials used as coatings, the following galvanization procedures are distinguished.
Copper plating
Galvanic coating with copper (copper sulfate) helps to strengthen metal products and improve their conductive qualities. Copper-plated metals are often used to make electrical conductors.
However, due to the fact that copper-plated parts do not resist corrosion well and oxidize over time, the copper plating process is most often intermediate and precedes the layering of another coating.
Chrome plating
Treating metals with chromium makes them more durable and resistant to aggressive environmental conditions, improves their appearance and restores damaged parts to their original parameters. Chromium forms a thin film on metal surfaces, which has not only protective, but also aesthetic qualities.
Depending on changes in the technological regime of chrome plating, a galvanic coating with different parameters and properties is obtained - matte gray (increases the hardness of the metal, but does not contribute to its wear resistance), shiny (provides wear resistance and hardness of products), milky plastic (promotes aesthetics, corrosion resistance of metals, but does not give them hardness).
Galvanizing
The most popular type of galvanization. A thin film of zinc on the surface of metals gives them shine and prevents the formation of rust. Galvanizing is especially popular in the automotive and construction industries. Zinc is used to process car body parts, pipe products, containers, roofing structures, including support structures.
Ironing
Used to enhance the strength of easily worn parts (for example, copper). The galvanized iron coating is practically resistant to corrosion.
Nickel plating
The use of this method of metal processing is optimal for making the metal material resistant to external environmental influences. The nickel layer reliably protects products or machine parts from rust formed under the influence of the external environment, as well as from types of corrosion resulting from contamination of the working environment by aggressive media - alkalis, acids, salts. Nickel-plated products demonstrate high resistance to severe mechanical damage and abrasion.
Gilding and silvering
They are often used in the electrical and radio-electronic industries and jewelry. Gold and silver give metal products a more presentable appearance, high reflective properties, protect them from adverse external factors, prevent corrosion, increase hardness and improve conductive qualities.
Tin electroplating
Tin gives metal parts strength and hardness. Galvanization with this material is used for aluminum, zinc, steel and copper.
Execution Goals
Electroplating can be applied to a metal surface for various purposes. For example, to perform galvanic chrome plating, the surface to be treated must be coated with a layer of nickel. Basically, galvanic coatings are applied in order to improve the protective properties and decorative characteristics of products. Electroplating is also used to create exact copies of parts that even have a very high complexity of relief. In such cases, the process is called galvanoplasty.
The method of galvanizing ferrous metals using galvanization is widely used. It allows you to form a layer of zinc on their surface, which is characterized by exceptionally high resistance to corrosion. Metal products processed using this technology can be used for a very long time in conditions of high humidity, in constant contact with fresh and salt water, without losing their original characteristics. Galvanizing is used, in particular, to process pipe products, various containers, and elements of roofing, building and supporting structures. Due to galvanizing, the metal receives not only barrier, but also electrochemical protection.
Galvanizing a car body in a galvanic bath
If with the help of galvanizing only the corrosion resistance of the metal is increased, then electroplating with chromium allows not only to solve this important problem, but also to make the surface of the workpiece harder and more wear-resistant, and also to increase its decorative appeal. Electroplated nickel coatings serve the same purposes.
Jewelry making is another area where electroplating plays a special role. Galvanization in this case is used to improve the decorative characteristics of the processed products. The electroplating process is used to coat a piece of jewelry with a layer of gold or silver, restoring a surface that has lost its appeal over time. It is noteworthy that even gold products are subjected to gilding using electroplating, which makes it possible to almost double the hardness of their surface layer. In addition, such a film applied to a gold product seems to illuminate it, making it brighter and more beautiful.
Other methods of protecting metals
In order to increase the strength and corrosion resistance of metals, in addition to galvanization, many other processing methods are used: hardening, recrystallization, embossing, rolling, flame spraying, surfacing, etc.
One of the simplest, most effective and fastest ways to ensure the strength and wear resistance of metal parts, to prevent their corrosion and destruction under the influence of aggressive external factors is the use of special anti-friction coatings. In appearance, they resemble paints, but instead of pigments they contain particles of solid lubricants.
Antifriction solid lubricant coatings form a thin, dry film on surfaces with a very low coefficient of friction and high load-bearing capacity. This is especially important for metal parts used in moving mechanisms exposed to extreme loads, pressures, and temperatures.
In Russia, such coatings are produced under the MODENGY brand.
When applied to the surface, they fill all micro-irregularities, thereby increasing its supporting area and load-bearing capacity. Thanks to dry (non-sticky) lubrication technology, MODENGY materials are functional in dusty environments. They are resistant to chemically aggressive substances, have high extreme pressure properties, and can withstand vacuum and radiation conditions.
produces more than 16 types of coatings containing solid lubricants of various chemical natures: molybdenum disulfide, polarized graphite, polytetrafluoroethylene (PTFE), tungsten disulfide, etc.