Everyone knows that diamond is currently the standard of hardness, i.e. When determining the hardness of a material, the hardness of a diamond is taken as a basis. In our article we will look at the ten hardest materials in the world and see how hard they are relative to diamond. A material is considered superhard if its indicators are above 40 GPa. It should be taken into account that the hardness of the material can fluctuate depending on external factors, in particular the load applied to it. So, we present the ten hardest materials in the world.
Lonsdaleite
Lonsdaleite is very similar in structure to diamond, because they are both allotropic modifications of carbon. Lonsdaleite was discovered in the crater of a meteorite, one of the components of which was graphite. Apparently, from the loads caused by the meteorite explosion, the graphite turned into lonsdaleite. When discovered, lonsdaleite did not demonstrate any special champion hardness indicators, but it was proven that if there were no impurities in it, it would be harder than diamond! Proven hardness of lonsdaleite is up to 152 GPa
Carbin
General information:
- Opening date: early 60s;
- Discoverers - Sladkov, Kudryavtsev, Korshak, Kasatkin;
- Density – 1.9-2 g/cm3.
Recently, scientists from Austria completed work on establishing the sustainable production of carbyne, which is an allotropic form of carbon based on sp-hybridization of carbon atoms. Its strength indicators are 40 times higher than those of diamond. Information about this was published in one of the issues of the scientific printed periodical “Nature Materials”.
The structure of carbine
After carefully studying its properties, scientists explained that its strength cannot be compared with any previously discovered and studied material. However, the production process encountered significant difficulties: the structure of carbyne is formed from carbon atoms collected in long chains, as a result of which it begins to break down during the manufacturing process.
To eliminate the identified problem, physicists from the public university in Vienna created a special protective coating in which carbyne was synthesized. Layers of graphene placed on top of each other and rolled into a “thermos” were used as a protective coating. While physicists worked hard to achieve stable forms, they discovered that the electrical properties of a material are affected by the length of the atomic chain.
Researchers have not learned how to extract carbyne from a protective coating without damage, so the study of the new material continues, scientists are guided only by the relative stability of atomic chains.
Carbin
Carbyne is a little-studied allotropic modification of carbon, the discoverers of which were Soviet chemists: A.M. Sladkov, Yu.P. Kudryavtsev, V.V. Korshak and V.I. Kasatochkin. Information about the results of the experiment with a detailed description of the discovery of the material in 1967 appeared on the pages of one of the largest scientific journals, “Reports of the USSR Academy of Sciences.” 15 years later, an article appeared in the American scientific journal Science that cast doubt on the results obtained by Soviet chemists. It turned out that the signals assigned to the little-studied allotropic modification of carbon could be associated with the presence of silicate impurities. Over the years, similar signals have been discovered in interstellar space.
Fullerite
It's time to look at the hardest substance in the world - fullerite. Fullerite is a crystal that consists of molecules rather than individual atoms. Thanks to this, fullerite has phenomenal hardness; it can easily scratch diamond, just like steel scratches plastic! The hardness of fullerite is 310 GPa.
Fullerite
We have provided a list of the hardest materials in the world at the moment. As we can see, among them there are enough substances harder than diamond and, perhaps, new discoveries await us ahead that will allow us to obtain materials with even higher hardness levels!
Wurtzite boron nitride
General information about boron nitride:
- Density – 2.18 g/cm3;
- Melting point – 2973 degrees Celsius;
- Crystal structure – hexagonal lattice;
- Thermal conductivity – 400 W/(m×K);
- Hardness – less than 10 units on the Mohs scale.
Wurtzite boron nitride
The main differences between wurtzite boron nitride, which is a compound of boron and nitrogen, are thermal and chemical resistance and fire resistance. The material can have different crystalline forms. For example, graphite is the softest, but at the same time stable, it is used in cosmetology. The sphalerite structure in the crystal lattice is similar to diamonds, but is inferior in terms of softness, while having better chemical and thermal resistance. Such properties of wurtzite boron nitride make it possible to use it in equipment for high-temperature processes.
Elbor
Elbor is also called kingsongite and borazon. This material is almost as hard as diamond. Due to this, it is widely used in the processing of various hard alloys. Elbor is a natural modification of boron nitride.
Elbor is the only boron compound that is formed in the depths of our planet. The remaining minerals, which contain boron, originate near the surface of the Earth.
Elbor was discovered in a part of the earth’s crust, which, during the evolution of the planet, seemed to “dive” under the neighboring lithospheric plate. At a depth of more than three hundred kilometers at a temperature of about 1200 degrees, chemical transformations occurred, as a result of which this superhard mineral appeared. This happened about 180 million years ago.
Maraging steel
General information:
- Impact strength of KST – 0.25-0.3 MJ/m2;
- Elastic limit – 1500 MPa;
- KCU – 0.4-0.6 MJ/m2.
Maraging steel
General information:
- Impact strength of KST – 0.25-0.3 MJ/m2;
- Elastic limit – 1500 MPa;
- KCU – 0.4-0.6 MJ/m2.
Maraging steels are iron alloys that have high impact strength without losing their ductility. Despite these characteristics, the material does not hold the cutting edge. Alloys obtained by heat treatment are low-carbon substances that take their strength from intermetallic compounds. The alloy contains nickel, cobalt and other carbide-forming elements. This type of high-strength, high-alloy steel is easy to process, due to the low carbon content in its composition. A material with such characteristics has found application in the aerospace field; it is used as a coating for missile casings.
The hardest mineral on Earth helps in space exploration
Diamond is also in demand in the chemical industry. An aggressive environment that easily damages glass is absolutely not dangerous for diamond. Physicists use crystals to conduct quantum physics experiments and explore outer space.
When creating telescope optics, the requirements for accuracy and reliability of materials become critical. This is where the hardest natural mineral, which has outstanding physical and chemical parameters, comes into play.
From kings to workers
For a long time, diamonds were the exclusive prerogative of jewelry makers. However, with the development of industry, this hardest mineral increasingly began to be considered not only from the usual aesthetic side, but also from the point of view of its unique physical properties. At first, natural diamonds that could not be cut were used in the production of instruments. These are stones that had defects that could not be corrected by a jeweler. They began to be called industrial diamonds.
As time passed, the need for tools with diamond cutting and drilling edges increased. For example, diamond drills are in great demand in the construction industry. Their advantage over their counterparts made from hard metal alloys is that when working with a diamond drill, microcracks do not form in the material. Diamond easily and cleanly cuts any material, be it stone, concrete or metal. And the absence of microcracks is the key to the durability of the structure. In addition, the work process itself is much faster, noticeably easier and much quieter.
Based on this, it is not surprising that, according to data for 2016, in Russia alone, 1,200 types of various tools and equipment are produced, the main working part of which is diamond.
About the natural properties of mineral rocks
Minerals are chemical native elements or compounds present in varying quantities in the depths of the earth's crust. Mineral compounds are part of soils, rocks and soil. Minerals are distributed very unevenly in nature.
Currently, the total number of their varieties and subspecies is over 3,000 thousand. Only 30-50 of them can be called the most famous and widespread. These are the main samples that received their own unique names.
There are many more chemical minerals in nature than there are minerals themselves. And recently, two types of substances have been classified as minerals:
- some components formed during the production of building materials, including ceramics, concrete and bricks;
- compounds of inorganic nature present in cosmetics, medicines and food products.
In nature, minerals are predominantly found in solid form. Much less often, mineral compounds are found in liquid form (as part of groundwater), as well as in the form of gases (methane and radon). The main part of solid mineral substances consists of crystals, as well as colloidal and amorphous compounds.
Externally, they are all very diverse and have many different, sometimes unique properties. It is noteworthy that the same chemical elements can crystallize into different structural formations, representing different types of minerals. This phenomenon in geology is usually called polymorphism.
Anisotropic as well as isotropic minerals are found in nature. The latter have the same properties in all directions. The former have properties that differ in a non-parallel direction. Mineral rocks also differ in the nature of their origin into exogenous (formed on the surface or seabed) and endogenous (originated in the deep interior of the earth's crust).
Future at $20 a skein
What is the hardest material that any average person can buy today? For just $20 you can buy 6 meters of Braeön tape. Since 2022, it has gone on sale from manufacturer Dustin McWilliams. The chemical composition and production method are kept strictly secret, but its qualities are amazing.
Absolutely anything can be secured with tape. To do this, you need to wrap it around the parts being fastened, heat it with a regular lighter, give the plastic composition the desired shape, and that’s it. After cooling, the joint will withstand a load of 1 ton.
Where are the most durable materials used?
Heavy-duty materials are used in many areas of life. Thus, chemists in Ireland and America have developed a technology by which durable textile fiber is produced. A thread of this material has a diameter of fifty micrometers. It is created from tens of millions of nanotubes, which are bonded together using a polymer.
Particularly durable textile materials are in demand
The tensile strength of this electrically conductive fiber is three times higher than that of the web of an orb-weaving spider. The resulting material is used to make ultra-light body armor and sports equipment. The name of another durable material is ONNEX, created by order of the US Department of Defense. In addition to its use in the production of body armor, the new material can also be used in flight control systems, sensors, and engines.
Special nanotubes make the materials especially durable
There is a technology developed by scientists, thanks to which strong, hard, transparent and lightweight materials are obtained through the transformation of aerogels. Based on them, it is possible to produce lightweight body armor, armor for tanks and durable building materials.
Novosibirsk scientists have invented a plasma reactor of a new principle, thanks to which it is possible to produce nanotubulene, a super-strong artificial material. This material was discovered twenty years ago. It is a mass of elastic consistency. It consists of plexuses that cannot be seen with the naked eye. The thickness of the walls of these plexuses is one atom.
Russian scientists have invented a super-reliable nanotubulene material
The fact that the atoms seem to be nested into each other according to the “Russian doll” principle makes nanotubulene the most durable material of all known. When this material is added to concrete, metal, and plastic, their strength and electrical conductivity are significantly enhanced. Nanotubulene will help make cars and planes more durable. If the new material comes into widespread production, then roads, houses, and equipment can become very durable. It will be very difficult to destroy them. Nanotubulene has not yet been introduced into widespread production due to its very high cost. However, Novosibirsk scientists managed to significantly reduce the cost of this material. Now nanotubulene can be produced not in kilograms, but in tons.
Nanotubulene has not yet found widespread use
Wonders of Wildlife
Among the living beings on our planet there are those who have something very special.
- Web of Caerostris darwini. The thread that Darwin's spider produces is stronger than steel and harder than Kevlar. It was this web that NASA scientists used when developing space protective suits.
- Teeth of the limpet mollusk - their fibrous structure is now being studied by bionics. They are so strong that they allow the mollusk to tear off algae that has grown into the stone.
Together we are stronger
One metal is good, but in some combinations it is possible to impart amazing properties to the alloy.
An ultra-strong alloy of titanium and gold is the only strong material that has been shown to be biocompatible with living tissue. The beta-Ti3Au alloy is so strong that it cannot be ground in a mortar. It is already clear today that this is the future of various implants, artificial joints and bones. In addition, it can be used in drilling, sports equipment and many other areas of our lives.
An alloy of palladium, silver and some metalloids may have similar properties. Scientists at the Caltec Institute are currently working on this project.
Indestructible Thermal Conductor
Translated from ancient Greek, the word “diamond” means “indestructible.” Even before antiquity, people knew the incredible strength of this stone. In ancient times, diamonds were widely traded in India and Egypt. And this mineral came to the European expanses after the aggressive campaigns of Alexander the Great. He brought the stones as magical artifacts. The ancient Greeks called this hardest mineral the tears of the gods that fell to the ground.
But the secret of the stone’s indestructibility lies, of course, not in mysticism or in connection with the spiritual world. The clear lattice structure of the element in the form of tetrahedrons and the strong bond between carbon atoms provide the highest strength. Thanks to this same structure, diamond is an excellent thermal conductor. For example, if it were possible to make a teaspoon from a single piece of diamond, you would not be able to stir sugar in hot tea with it, because you would get burned the moment the spoon touched the boiling water.
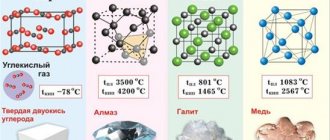
Characteristics of the hardness of mineral rocks
The hardness of minerals reflects the degree of resistance of a mineral sample to external mechanical influences from other, including harder materials. This property is determined by the structure and strength characteristics of the crystal lattice, its structure, the strength of chemical bonds, the nature, as well as the charge and size of the particles.
Hardness is also influenced by some mechanical parameters, including plasticity, elasticity, brittleness, as well as density, interatomic distance and the presence of dislocations. Crystals of most mineral rocks are characterized by anisotropy in hardness. The transition to a metamict state and hydration contribute to a decrease in this indicator.