Iron as a material became known from 3–4 thousand BC. e. At first, meteorite iron came to the attention of people, so in those days it was valued higher than gold. Then the Hittites mastered the development of sedimentary deposits, and the Romans learned to smelt cast iron.
Since then, the scope of metal use has only expanded. And so today we will talk about the use of iron and its compounds in human life: in everyday life, the national economy, industry and the use of metal in other areas.
Iron, properties of the atom, chemical and physical properties.
Fe 26 Iron
55.845(2) 1s2 2s2 2p6 3s2 3p6 3d6 4s2
Iron is an element of the periodic system of chemical elements of D.I. Mendeleev with atomic number 26. It is located in the 8th group (according to the old classification - a secondary subgroup of the eighth group), the fourth period of the periodic system.
Iron atom and molecule. Iron formula. Structure of the iron atom
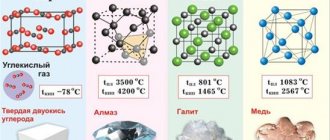
Isotopes and modifications of iron
Properties of iron (table): temperature, density, pressure, etc.
Physical properties of iron
Chemical properties of iron. Interaction of iron. Chemical reactions with iron
Getting iron
Application of iron
Table of chemical elements D.I. Mendeleev
Where is it used?
It’s hard to imagine everyday life without metal: iron is practical, reliable, and cheap. Perhaps someday it will be replaced by plastic. Today the advantages of iron material are at a premium.
Ultrapure iron
Industry
Iron has found uses in all forms. Alloys are the basis of materials in demand by industry. Powder is purchased in tons for welding, pyrotechnics, and printers. Compounds - the basis of mineral paints, pigments in the production of textiles and inks.
No industry can do without it:
- Mechanical engineering. The body of machines and mechanisms, especially for working in extreme conditions.
- Construction. Load-bearing structures of buildings, structures (bridges, mobile communication towers, etc.), fittings. Roofing material, corrugated sheets, metal tiles.
- Electrical engineering. Cores of electromagnets, armatures of electric machines, battery plates.
- Communications. Industrial and domestic pipelines for pumping steam, water, gas, and oil are made of steel and cast iron. This is the sheath of power cables.
Iron is the anode in iron-nickel, iron-air batteries. Household and professional tools are made from steel.
Other areas
Metal is used in science, medicine, and everyday life:
- Cleaning of drains.
- Component of gart (printing font).
- Kitchen utensils, tableware.
- Doors, locks.
- Ultra-fine magnetite (metal oxide) powder is used to fill black-and-white printers.
- Furniture of avant-garde styles.
- Anemia is treated with iron supplements.
- Gardeners and builders destroy the fungus with a mixture of copper and iron sulfate (septate metal sulfate).
Artificial radioactive isotopes are a marker in the analysis of chemical, technological and biological processes.
Iron atom and molecule. Iron formula. Structure of the iron atom:
Iron (lat. Ferrum) is a chemical element of the periodic system of chemical elements of D.I. Mendeleev with the designation Fe and atomic number 26. It is located in the 8th group (according to the old classification - a secondary subgroup of the eighth group), the fourth period of the periodic system.
Iron is a metal. Belongs to the group of transition metals. Refers to ferrous metals .
Iron is represented by the symbol Fe.
As a simple substance , iron under normal conditions is a malleable, viscous metal of a silvery-white color with a grayish tint with high chemical reactivity. Iron itself is usually called its alloys with a low impurity content (up to 0.8%), which retain the softness and ductility of pure metal. In practice, alloys of iron with carbon are more often used: steel (up to 2.14 wt.% carbon) and cast iron (more than 2.14 wt.% carbon), as well as stainless (alloy) steel with additions of alloying metals (chrome, manganese, nickel and etc.).
The iron molecule is monatomic.
Chemical formula of iron is Fe.
The electronic configuration of the iron atom is 1s2 2s2 2p6 3s2 3p6 3d6 4s2. The ionization potential (first electron) of the iron atom is 762.47 kJ/mol (7.9024681(12) eV).
The structure of the iron atom. The iron atom consists of a positively charged nucleus (+26), around which 26 electrons move in four shells. In this case, 24 electrons are in the internal level, and 2 electrons are in the external level. Since iron is located in the fourth period, there are only four shells. First, the inner shell is represented by the s-orbital. The second – the inner shell is represented by s- and p-orbitals. The third - inner shell is represented by s-, p- and d-orbitals. The fourth - outer shell is represented by the s-orbital. At the internal energy level of the iron atom, the 3d orbital contains two paired and four unpaired electrons. At the outer energy level of the iron atom, the s orbital contains two paired electrons. In turn, the nucleus of an iron atom consists of 26 protons and 30 neutrons.
The radius of the iron atom (calculated) is 156 pm.
The atomic mass iron atom is 55.845(2) a. eat.
Iron , one of the most common metals in the earth's crust, ranks fourth. The iron content in the earth's crust is 6.3% (by weight). In this indicator, iron is second only to oxygen, silicon and aluminum.
Iron, atomic properties, chemical and physical properties
Receiving technology
Iron ore (magnetite and hematite) is sent to work: to a processing or metallurgical plant.
Iron ore
Pig iron is smelted in a blast furnace. At 1610°C, the mixture (sinter, pellets) with flux is loaded and blown with hot air. This allows you to remove impurities and separate slag.
The main methods of producing steel:
- Martenovsky. Molten cast iron, ore, and scrap are melted at 2100°C.
If necessary, alloying additives are added at the end of the melt.
- Oxygen converter . The cast iron mass in the furnace is blown with air under pressure. A mixture of oxygen and air or pure oxygen is used (for steels with premium characteristics).
- Electric melting. Cast iron is burned in an electric furnace at 2250°C. The method is used for smelting alloy and other special grades of steel.
- Straight. The iron-rich pellets are loaded into the furnace. Blow with hydrogen at 1050°C.
Hardening steel—heating until hot and cooling—makes it ductile and hard.
The production of pure metal is based on the electrolysis of molten salts of the substance.
An iron alloy containing less than 2% carbon is steel. More than 2% carbon is cast iron.
Application
More than 90% of all metallurgical production is occupied by iron and its alloys.
Products made from steels and cast irons are an irreplaceable and major part of structural materials, including buildings, bridges, railways and much more.
Application of iron compounds:
- Divalent and trivalent iron is used as a coagulant in water treatment systems;
- the anodes in iron-nickel and iron-air batteries are made of the most famous ferrous metal;
- magnetite in the form of ultrafine powder is used in black and white laser printers;
- FeCl3 is used by radio amateurs (etching printed circuit boards);
- Magnetite is indispensable in the manufacture of memory media (hard drives).
Areas of application of iron
For most organisms, there is no life without iron; with its help, oxygen is delivered to every cell of the body. Lack of iron leads to chlorosis in plants and iron deficiency anemia in animals.
Cognitive: the belief that an apple when cut is darkened by the iron contained in it is a myth.
Where do they come from?
There are four natural suppliers of heavy metals:
- Mountain raw materials . Most often these are igneous or sedimentary rocks.
- Rock-forming minerals . For copper, for example, it is malachite and other minerals.
- Volcanoes. Particles of matter erupt along with volcanic products (gases, geysers).
Malachite stone
Another source is the Universe. The substance is carried into the stratosphere by meteorites or clouds of cosmic dust.
How they searched for iron ore
In the middle of the century, metal products were highly valued, they were cherished and also passed on by inheritance.
The path to becoming a cauldron or an ax in those days was very long and long: iron had to be found and then processed.
The business began with a search for places where metal ores lay. The search was helped by the experience that people have accumulated over many centuries. First of all, these are deposits that reach the surface of the earth.
Throughout Europe, iron was found in the form of lumps of ore:
1. greenish - at the bottom of lakes;
2. reddish - under the turf;
3. reddish - in forest swamps.
The bottom of the clear lakes was scanned from boats, or they dived into the muddy water in search of pieces of ore, which they scooped out with scoops.
Iron ore was also found in brown vegetation. The meadow turf was cut, the swamp layers were torn off, and the ore nest was taken out with shovels. Sometimes such a meadow was covered with thousands of holes.
A little later, ore began to be mined in mines that reached depths of up to 500 meters.
Iron ore was lifted from the mines using lifting mechanisms, and groundwater was pumped out with hand pumps.
History of steel production
BC. Wrought iron was already being produced everywhere in Europe. Many magnificent Greek and Roman buildings were built of stone using butterfly-shaped iron tools covered with lead. In 500 BC. e. The Etruscans who lived on the west coast of Italy produced more than 4.5 thousand kilograms of iron per year.
Iron was forged in a forge, and charcoal was used to keep the fire going. The fire was fanned using special bellows made from animal skins. Later, the small stone furnaces were dismantled and mass iron smelting began. Ore was delivered to the furnaces on sailing ships. Due to the fact that the ore processing method used by the Etruscans was ineffective, its reserves were quickly depleted. In addition, charcoal production has sharply reduced the number of forests in western Italy.
The first steel was created by the Celts around 200 AD. e. They cut the wrought iron into thin strips and placed them in a container with burnt bones and charcoal, after which they heated the whole thing in a furnace for 10-12 hours at very high heat. As a result, the metal surface was enriched with carbon. Then they welded these strips together through forging and thus created knives. These knives became the predecessors of the blades that we mistakenly call Damascus.
The Celtic process for making steel in 1050 was copied by the Vikings and Germans. Since then, steel blades have been produced in these countries, the manufacturing method of which was strictly classified. Damascus steel was produced in Pakistan and sent in the form of damask steel to Syria, where the famous Damascus blades were made. The process of producing Damascus steel is very complex because it had to be heated to a very high temperature, and if the temperature was exceeded, the material could break.
Over time, the melting temperature of iron in furnaces became higher and higher, so the resulting iron contained 3-4% carbon.
It was fragile and suitable only for casting. It was impossible to make knives and parts for transport from it. In addition, by this time a huge part of the forests in Europe had been cut down for construction purposes and the production of charcoal.
Then the King of England issued a decree that no more forests could be cut down, and steel producers had to come up with a way to process coal into coke. In England, they developed a method for tinning steel by mixing molten iron with iron silicate and iron oxide. Iron silicate is one of the components of wrought iron.
Coal-fired furnaces were called the furnace. One worker had to stir the resulting mixture, which created carbon dioxide, so the melting point of the iron became higher, and the tinning process began.
Large pieces weighing from 90 kg to 130 kg were placed inside. Another worker, using a pair of large tongs, took these pieces and placed them under a press to squeeze out the iron silicate from them. After the press, the pieces were placed in a rolling mill, where they were formed into strips of cast iron.
These strips were cut into short pieces and joined together, after which they were placed in a recess filled with carbon and heated to welding temperature. After this, the strips of red iron were again sent to the rolling mill and graded iron was obtained. This method was used not only in Europe, but in the eastern United States.
To make steel, thin rolled products were placed in a cavity filled with carbon obtained from burning bones and heated at high temperatures for several days.
The carbon was absorbed by the iron, resulting in bubbly steel. Cement steel or tomlenka was called bubbly. This concept arose from the appearance of the strips recovered from the carbon pit, which were covered in bubbles. After this, the strips were folded together and forged, then folded again and forged, in this way high-quality steel was obtained.
England needed high-quality steel to create a fleet that could cross the ocean.
One enterprising Englishman noticed that glassblowers could achieve very high temperatures in their furnaces. He took strips of bubbled steel and placed them in a ceramic crucible, then placed the container in the glassblowing furnace. As a result, the steel melted, the iron silicate evaporated, but the carbon remained, resulting in very high quality steel. At that time, many people were watching the process, and he could not keep it a secret.
This method produced cast steel, from which a large number of old tools were made in the United States, labeled "cast steel."