The beginning of the history of iron
In the third millennium BC. e. people began to mine and learned to process bronze and copper. They were not widely used due to their high cost. The search for new metal continued. The history of iron began in the first century BC. e. In nature, it can only be found in the form of compounds with oxygen. To obtain pure metal, it is necessary to separate the last element. It took a long time to melt the iron, since it had to be heated to 1539 degrees. And only with the advent of cheese-making furnaces in the first millennium BC did they begin to obtain this metal. At first it was fragile and contained a lot of waste.
With the advent of forges, the quality of iron improved significantly. It was further processed in a blacksmith, where the slag was separated by hammer blows. Forging has become one of the main types of metal processing, and blacksmithing has become an indispensable branch of production. Iron in its pure form is a very soft metal. It is mainly used in an alloy with carbon. This additive enhances the physical property of iron, such as hardness. The cheap material soon penetrated widely into all spheres of human activity and revolutionized the development of society. After all, even in ancient times, iron products were covered with a thick layer of gold. It had a high price compared to the noble metal.
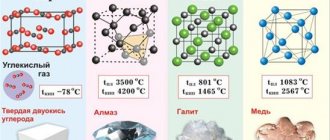
Atomic crystal lattice
Substances with an atomic crystal lattice, as a rule, have strong covalent bonds in their nodes consisting of atoms themselves. A covalent bond occurs when two identical atoms share fraternal electrons with each other, thus forming a common pair of electrons for neighboring atoms. Because of this, covalent bonds bind atoms tightly and evenly in a strict order - perhaps this is the most characteristic feature of the structure of the atomic crystal lattice. Chemical elements with similar bonds can boast of their hardness and high melting point. Chemical elements such as diamond, silicon, germanium, and boron have an atomic crystal lattice.
Iron in nature
The lithosphere contains more aluminum than iron. In nature, it can only be found in the form of compounds. Ferric iron, reacting, turns the soil brown and gives the sand a yellowish tint. Iron oxides and sulfides are scattered in the earth's crust, sometimes there are accumulations of minerals, from which the metal is subsequently extracted. The content of ferrous iron in some mineral springs gives the water a special taste.
Rusty water flowing from old water pipes is colored by the trivalent metal. Its atoms are also found in the human body. They are found in hemoglobin (iron-containing protein) in the blood, which supplies the body with oxygen and removes carbon dioxide. Some meteorites contain pure iron, sometimes whole ingots are found.
Crystal lattices, video
And finally, a detailed video explanation about the properties of crystal lattices.
Author: Pavel Chaika, editor-in-chief of Poznavaika magazine
When writing the article, I tried to make it as interesting, useful and high-quality as possible. I would be grateful for any feedback and constructive criticism in the form of comments on the article. You can also write your wish/question/suggestion to my email [email protected] or Facebook, with respect, the author.
Popular science magazine Poznavaika
This article is available in English - Crystal Lattice in Chemistry.
What physical properties does iron have?
It is a ductile silver-white metal with a grayish tint and a metallic sheen. It is a good conductor of electric current and heat. Due to its ductility, it lends itself perfectly to forging and rolling. Iron does not dissolve in water, but liquefies in mercury, melts at a temperature of 1539 and boils at 2862 degrees Celsius, and has a density of 7.9 g/cm³. A peculiarity of the physical properties of iron is that the metal is attracted by a magnet and, after the cancellation of the external magnetic field, retains magnetization. Using these properties, it can be used to make magnets.
Metal crystal lattice
The type of bond of a metal crystal lattice is more flexible and ductile than the ionic one, although in appearance they are very similar. Its distinctive feature is the presence of positively charged cations (metal ions) at lattice sites. Between the nodes live electrons that participate in the creation of the electric field; these electrons are also called electric gas. The presence of such a structure of a metal crystal lattice explains its properties: mechanical strength, heat and electrical conductivity, fusibility.
Chemical properties
Iron has the following properties:
- in air and water it easily oxidizes, becoming covered with rust;
- in oxygen, the hot wire burns (and scale is formed in the form of iron oxide);
- at a temperature of 700–900 degrees Celsius, it reacts with water vapor;
- when heated, reacts with non-metals (chlorine, sulfur, bromine);
- reacts with dilute acids, resulting in iron salts and hydrogen;
- does not dissolve in alkalis;
- is capable of displacing metals from solutions of their salts (an iron nail in a solution of copper sulfate becomes covered with a red coating - this is the release of copper);
- In concentrated alkalis when boiling, the amphotericity of iron is manifested.
Atomic crystal lattice
The nodes contain atoms, as the name suggests. These substances are very strong and durable , since a covalent bond is formed between the particles. Neighboring atoms share a pair of electrons with each other (or rather, their electron clouds are layered on top of each other), and therefore they are very well connected to each other. The most obvious example is diamond, which has the greatest hardness on the Mohs scale. Interestingly, diamond, like graphite, consists of carbohydrates. Graphite is a very brittle substance (Mohs hardness 1), which is a clear example of how much depends on the type.
Atomic region lattice is poorly distributed in nature, it includes: quartz, boron, sand, silicon, silicon (IV) oxide, germanium, rock crystal. These substances are characterized by a high melting point, strength, and these compounds are very hard and insoluble in water. Due to the very strong bonds between atoms, these chemical compounds hardly interact with others and conduct current very poorly.
Feature properties
One of the physical properties of iron is ferromagneticity. In practice, the magnetic properties of this material are often encountered. This is the only metal that has such a rare feature.
Under the influence of a magnetic field, iron is magnetized. The metal retains its formed magnetic properties for a long time and remains a magnet itself. This exceptional phenomenon is explained by the fact that the structure of iron contains a large number of free electrons that can move.
Crystal structure
MATERIALS SCIENCE
Substances can be in three states of aggregation: solid, liquid and gaseous. The transition from a solid to a gaseous state is called sublimation.
All metals are crystalline solids.
Each metal is characterized by its own spatial crystal lattice with long-range order in the arrangement of atoms (a certain arrangement of atoms at any distance).
In solids, the order of arrangement of atoms is natural. The arrangement of atoms can be represented in the form of elementary crystalline cells. There are 14 types of gratings in total: 3 types are typical for metals:
Reserves and production
One of the most common elements on earth is iron. In terms of content in the earth's crust, it ranks fourth. There are many known ores that contain it, for example, magnetic and brown iron ore. The metal is produced in industry mainly from hematite and magnetite ores using the blast furnace process. First, it is reduced with carbon in a furnace at a high temperature of 2000 degrees Celsius.
To do this, iron ore, coke and flux are fed into the blast furnace from above, and a stream of hot air is injected from below. A direct process for obtaining iron is also used. The crushed ore is mixed with special clay to form pellets. Next, they are fired and treated with hydrogen in a shaft furnace, where it is easily restored. They obtain solid iron and then melt it in electric furnaces. Pure metal is reduced from oxides using electrolysis of aqueous salt solutions.
Aluminum strength
The annealed fraction of technical aluminum at room temperature has a tensile strength of up to 8 kg/mm2. Increasing the purity of the material increases its ductility, but is reflected in a decrease in strength. An example is aluminum, which is 99.99% pure. In this case, the ultimate strength of the material reaches about 5 kg/mm2.
A decrease in the tensile strength of an aluminum dough piece is observed when it is heated during tensile tests. In turn, lowering the metal temperature in the range from +27 to -260°C temporarily increases the test indicator by 4 times, and when testing the highest purity aluminum fraction - by as much as 7 times. At the same time, the strength of aluminum can be slightly increased by alloying it.
Benefits of Iron
The basic physical properties of the iron substance give it and its alloys the following advantages over other metals:
- They have hardness and strength while maintaining elasticity. These qualities are not the same for different alloys and depend on alloying additives, production methods and heat treatment.
- A wide variety of cast iron and steels allow them to be used for any needs in the national economy.
- The high magnetic properties of metal are indispensable for the manufacture of magnetic cores.
- The feasibility of light mechanical processing, due to the physical properties of iron, makes it possible to obtain sheets, rods, beams, pipes, and shaped profiles from its alloys.
- The significant malleability of the material allows it to be used for decorative products.
- Low cost of alloys.
Crystal structure of metals. Types of crystal lattices
When a metal forms a solid structure, all its atoms tend to occupy such positions in space relative to each other that they correspond to a minimum of potential energy. This minimum corresponds to the crystal lattice.
A crystal lattice is understood as a spatial atomic structure that can be obtained if the coordinates of a limited number of its atoms and the vector of their translation in space are known. The specified number of atoms is called the lattice basis, and their positions form the so-called unit cell.
All metals crystallize in three main types of lattices:
- face-centered cubic (fcc);
- body-centered cubic (bcc);
- hexagonal close-packed (hcp).
Due to their crystalline structure, metals have properties such as plasticity, elasticity and metallic luster.
Flaws
In addition to a large number of positive qualities, there are also a number of negative properties of the metal:
- Products are susceptible to corrosion. To eliminate this undesirable effect, stainless steels are produced by alloying, and in other cases, special anti-corrosion treatment is carried out on structures and parts.
- Iron accumulates static electricity, so products containing it are subject to electrochemical corrosion and also require additional processing.
- The specific gravity of the metal is 7.13 g/cm³. This physical property of iron gives structures and parts increased weight.
Composition and structure
Iron has four crystalline modifications that differ in structure and lattice parameters. For the smelting of alloys, it is the presence of phase transitions and alloying additives that is of significant importance. The following states are distinguished:
- Alpha phase. It lasts up to 769 degrees Celsius. In this state, iron retains the properties of a ferromagnet and has a body-centered cubic lattice.
- Beta phase. Exists at temperatures from 769 to 917 degrees Celsius. It has slightly different lattice parameters than in the first case. All physical properties of iron remain the same, with the exception of magnetic ones, which it loses.
- Gamma phase. The lattice structure becomes face-centered. This phase appears in the range of 917–1394 degrees Celsius.
- Omega phase. This state of the metal appears at temperatures above 1394 degrees Celsius. It differs from the previous one only in the lattice parameters.
Iron is the most sought after metal in the world. More than 90 percent of all metallurgical production falls on it.
Copper strength
Under normal conditions at room temperature, annealed commercial copper has a tensile strength of about 23 kg/mm2. With significant temperature loads on the material, its ultimate strength is significantly reduced. The indicators of the ultimate strength of copper are reflected by the presence of various impurities in the metal, which can both increase this indicator and lead to its decrease.
Application
People first began to use meteorite iron, which was valued higher than gold. Since then, the scope of this metal has only expanded. The following are the uses of iron based on its physical properties:
- ferromagnetic oxides are used for the production of magnetic materials: industrial installations, refrigerators, souvenirs;
- iron oxides are used as mineral paints;
- ferric chloride is indispensable in amateur radio practice;
- Ferrous sulfates are used in the textile industry;
- magnetic iron oxide is one of the important materials for the production of long-term computer memory devices;
- ultrafine iron powder is used in black and white laser printers;
- the strength of the metal makes it possible to manufacture weapons and armor;
- wear-resistant cast iron can be used to produce brakes, clutch discs, and parts for pumps;
- heat-resistant – for blast furnaces, thermal furnaces, open-hearth furnaces;
- heat-resistant – for compressor equipment, diesel engines;
- high-quality steel is used for gas pipelines, casings of heating boilers, dryers, washing machines and dishwashers.