Lead is a metal that can also be considered one of the metals known since ancient times. It is believed that its smelting was the first metallurgical process in human history. Over the past millennia, lead was used little, then again “came into fashion,” but was never forgotten.
The reason for this is its interesting qualities. And today we will study the physicochemical, mechanical and magnetic properties, technical characteristics of lead, its alloys and oxides, review photos of the element and give useful tips on its use.
General information:
| 100 | General information | |
| 101 | Name | Lead |
| 102 | Former name | |
| 103 | Latin name | Plumbum |
| 104 | English name | Lead |
| 105 | Symbol | Pb |
| 106 | Atomic number (number in table) | 82 |
| 107 | Type | Metal |
| 108 | Group | Amphoteric, heavy, non-ferrous metal |
| 109 | Open | Known since ancient times. |
| 110 | Opening year | 7000 BC |
| 111 | Appearance, etc. | Malleable, relatively fusible, heavy metal of silver-white color with a bluish tint |
| 112 | Origin | Natural material |
| 113 | Modifications | |
| 114 | Allotropic modifications | |
| 115 | Temperature and other conditions for the transition of allotropic modifications into each other | |
| 116 | Bose-Einstein condensate | |
| 117 | 2D materials | |
| 118 | Content in the atmosphere and air (by mass) | 0 % |
| 119 | Content in the earth's crust (by mass) | 0,00099 % |
| 120 | Content in seas and oceans (by mass) | 3,0·10-9 % |
| 121 | Content in the Universe and space (by mass) | 1,0·10-6 % |
| 122 | Abundance in the Sun (by mass) | 1,0·10-6 % |
| 123 | Content in meteorites (by mass) | 0,00014 % |
| 124 | Content in the human body (by weight) | 0,00017 % |
Historical information
Lead has been used for thousands of years because it is widespread and easy to mine and process. It is very malleable and melts easily. Lead smelting was the first metallurgical process known to man. Lead beads dating back to 6400 BC. BC, were found in the Çatalhöyük culture. The oldest object made of lead is often considered to be a figurine of a standing woman in a long skirt from the First Dynasty of Egypt, dating back to 3100-2900. BC BC, stored in the British Museum (stock number EA 32138). It was found in the Temple of Osiris at Abydos and brought from Egypt in 1899. In Ancient Egypt, lead medallions were used. In the Early Bronze Age, lead was used along with antimony and arsenic. Lead is referred to as a specific metal in the Old Testament.
Lead pipes of an ancient Roman water supply with inscriptions
The largest producer of lead in the pre-industrial era was Ancient Rome, with an annual production of 80,000 tons. Roman mining of lead took place in Central Europe, Roman Britain, the Balkans, Greece, Asia Minor and Spain. The Romans widely used lead in the production of pipes for water supply systems, and lead pipes often bore the inscriptions of Roman emperors. True, even Pliny and Vitruvius believed that this was not good for public health.
Papal bull of 1637 with lead seal
After the fall of the Roman Empire in the 5th century. n. e. Lead use in Europe fell and remained low for about 600 years. Then lead began to be mined in eastern Germany. Lead sugar has been added to wine since Roman times to improve its taste; this became widespread and continued even after the ban by a papal bull in 1498. This use of lead in the Middle Ages led to epidemics of lead colic. In Ancient Rus', lead was used to cover the roofs of churches, and was also widely used as a material for hanging seals on letters. Later, in 1633, a water supply system with lead pipes was built in the Kremlin, through which water came from the Vodovzvodnaya Tower; it existed until 1737.
In alchemy, lead was associated with the planet Saturn and was represented by its symbol ♄. In ancient times, tin, lead and antimony were often not distinguished from each other, considering them to be different types of the same metal, although Pliny the Elder distinguished between tin and lead, calling tin “plumbum album” and lead “plumbum nigrum”.
The Industrial Revolution led to a new increase in the demand for lead. By the beginning of the 1840s. annual production of refined lead exceeded 100,000 tons for the first time and grew to over 250,000 tons over the next 20 years. Until the last decades of the 19th century, lead mining was primarily carried out by three countries: Britain, Germany and Spain. By the beginning of the 20th century, lead mining in Europe had become less than in the rest of the world, thanks to increased production in the United States, Canada, Mexico and Australia. Until 1990, large quantities of lead were used (together with antimony and tin) to cast typographical fonts, and also in the form of tetraethyl lead to increase the octane number of motor fuels.
Chemical properties of lead:
| 300 | Chemical properties | |
| 301 | Oxidation states | -4, -2, -1, 0, +1, +2, +3, +4 |
| 302 | Valence | II, IV |
| 303 | Electronegativity | 2.33 (Pauling scale) |
| 304 | Ionization energy (first electron) | 715.6 kJ/mol (7.4166799(6) eV) |
| 305 | Electrode potential | Pb2+ + 2e— → Pb, Eo = -0.126 V, Pb4+ + 4e— → Pb, Eo = +0.77 V, Pb4+ + 2e— → Pb2+, Eo = +1.694 V |
| 306 | Electron affinity energy of an atom | 34.418 3(3) kJ/mol (0.356721(2) eV) |
Environmental characteristics
Lead pollution of the environment is considered one of the most dangerous. All products that use lead require special disposal, which is carried out only by licensed services.
Unfortunately, lead pollution is caused not only by the activities of enterprises, where it is at least regulated. In city air, the presence of lead vapor ensures the combustion of fuel in cars. Against this background, the presence of lead stabilizers in such familiar structures as a metal-plastic window no longer seems worth attention.
Lead is a metal of industrial importance. Despite its toxicity, it is used too widely in the national economy for the metal to be replaced with anything.
This video will tell you about the properties of lead salts:
Physical properties of lead:
| 400 | Physical properties | |
| 401 | Density* | 11.34 g/cm3 (at 20 °C and other standard conditions , state of matter – solid), 10.66 g/cm3 (at melting point 327.46 °C and other standard conditions , state of matter – liquid) |
| 402 | Melting temperature* | 327.46 °C (600.61 K, 621.43 °F) |
| 403 | Boiling temperature* | 1749 °C (2022 K, 3180 °F) |
| 404 | Sublimation temperature | |
| 405 | Decomposition temperature | |
| 406 | Self-ignition temperature of a gas-air mixture | |
| 407 | Specific heat of fusion (enthalpy of fusion ΔHpl) | 4.77 kJ/mol |
| 408 | Specific heat of evaporation (enthalpy of boiling ΔHboiling)* | 179.5 kJ/mol |
| 409 | Specific heat capacity at constant pressure | |
| 410 | Molar heat capacity | 26.65 J/(K mol) |
| 411 | Molar volume | 18.2716 cm³/mol |
| 412 | Thermal conductivity | 35.3 W/(m K) (at standard conditions ), 35.3 W/(m K) (at 300 K) |
| 413 | Thermal expansion coefficient | 28.9 µm/(MK) (at 25 °C) |
| 414 | Thermal diffusivity coefficient | |
| 415 | Critical temperature | |
| 416 | Critical pressure | |
| 417 | Critical Density | |
| 418 | Triple point | |
| 419 | Vapor pressure (mmHg) | |
| 420 | Vapor pressure (Pa) | |
| 421 | Standard enthalpy of formation ΔH | |
| 422 | Standard Gibbs energy of formation ΔG | |
| 423 | Standard entropy of matter S | |
| 424 | Standard molar heat capacity Cp | |
| 425 | Enthalpy of dissociation ΔHdiss | |
| 426 | The dielectric constant | |
| 427 | Magnetic type | |
| 428 | Curie point | |
| 429 | Volume magnetic susceptibility | |
| 430 | Specific magnetic susceptibility | |
| 431 | Molar magnetic susceptibility | |
| 432 | Electric type | |
| 433 | Electrical conductivity in the solid phase | |
| 434 | Electrical resistivity | |
| 435 | Superconductivity at temperature | |
| 436 | Critical magnetic field of superconductivity destruction | |
| 437 | Prohibited area | |
| 438 | Charge carrier concentration | |
| 439 | Mohs hardness | |
| 440 | Brinell hardness | |
| 441 | Vickers hardness | |
| 442 | Sound speed | |
| 443 | Surface tension | |
| 444 | Dynamic viscosity of gases and liquids | |
| 445 | Explosive concentrations of gas-air mixture, % volume | |
| 446 | Explosive concentrations of a mixture of gas and oxygen, % volume | |
| 446 | Ultimate tensile strength | |
| 447 | Yield strength | |
| 448 | Elongation limit | |
| 449 | Young's modulus | |
| 450 | Shear modulus | |
| 451 | Bulk modulus of elasticity | |
| 452 | Poisson's ratio | |
| 453 | Refractive index |
Metal mining by country of the world
Many countries around the world are actively mining Plumbum. The leading producer of the metal is China (half of all world production). Next come:
- Australia
- Peru
- Mexico
- India
At one time, the United States mined about a third of the world's total reserves, but as of 2022, the production of the gray heavy metal in this country is no more than 7% of global production.
Mineral ore - galena, the structure of which traditionally contains a fairly large amount of lead compared to other types of ore
Most of the metal mined in the United States comes from Alaska and Missouri, with slightly less from Idaho and Washington. About 60% of the metal used in America comes from “reclaimed” (recycled) sources.
In Russia, the level of production is approximately comparable to American production (about 5%). However, Russian production was only slightly behind China during the Soviet era. That is, from the point of view of reserves, a rich resource exists in Russia.
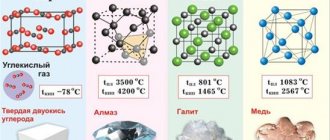
Lead crystal lattice:
| 500 | Crystal cell | |
| 511 | Crystal grid #1 | |
| 512 | Lattice structure | Cubic face centered |
| 513 | Lattice parameters | 4.950 Å |
| 514 | c/a ratio | |
| 515 | Debye temperature | 88K |
| 516 | Name of space symmetry group | Fm_3m |
| 517 | Symmetry space group number | 225 |
What is gray “heavy” metal used for?
The considered physical and chemical beneficial properties of lead allow us to some extent predict what this metal can be used for:
Protection
The density and heaviness properties of the metal are very useful as a material to protect people from harmful radiation (for example, radiologists using lead shields or aprons).
Coloring
Bright, durable compounds are well suited for pigments and dyes. True, due to the threat to human health, lead had to be removed from many paints (especially the paints of children's toys).
Plumbing
The properties of successfully resisting corrosion have made the metal an excellent material for the construction of roofing and plumbing. However, again due to health and safety concerns, many lead water pipes had to be removed and replaced with plastic ones.
Weapon
The weight of the metal proved useful for the production of small arms bullets. But this area, again, looks at the issue of concerns about health effects. Absurd, but true.
Batteries
Despite its weak electrical conductivity properties, lead is used in conjunction with sulfuric acid to store and release electrical energy as a result of a chemical reaction. This is the principle on which car batteries work.
Alloys
Important lead alloys include tin (used to make cookware), anti-corrosion coatings for electrical cables, acid-resistant gaskets for chemical tanks, and solders with relatively low melting points.
Prevalence in nature
The content of S in the earth's crust is 1.3·10–3% by mass, in ocean waters 0.03 μg/dm3, in river waters 0.2–8.7 μg/dm3. Natural background in the atmosphere is 2·10-9–5·10-4 µg/m3. The adult human body contains 7–15 mg Pb. Known approx. 80 minerals containing Pb; prom. galena PbS, anglesite PbSO4, cerussite PbCO3, pyromorphite Pb5(PO4)3Cl are of importance; see also Lead-zinc ores. Where lead ores occur, soil, plants and water contain up to 1% Pb.
§18. Magnetic properties of various substances
Ferromagnetic, paramagnetic and diamagnetic materials. All substances - solid, liquid and gaseous, depending on their magnetic properties, are divided into three groups: ferromagnetic, paramagnetic and diamagnetic.
Ferromagnetic materials include iron, cobalt, nickel and their alloys. They have high magnetic permeability, thousands and even tens of thousands of times greater than the magnetic permeability of non-ferromagnetic substances, and are well attracted to magnets and electromagnets.
Paramagnetic materials include aluminum, tin, chromium, manganese, platinum, tungsten, solutions of iron salts, etc. Relative magnetic permeability? they have slightly more than one. Paramagnetic materials are attracted to magnets and electromagnets thousands of times weaker than ferromagnetic materials.
Diamagnetic materials are not attracted to magnets, but, on the contrary, are repelled. These include copper, silver, gold, lead, zinc, resin, water, most gases, air, etc. Relative magnetic permeability? they have slightly less than one.
Magnetic properties of ferromagnetic materials. Ferromagnetic materials, due to their ability to be magnetized, are widely used in the manufacture of electrical machines, devices and other electrical installations. Their main characteristics are: the magnetization curve, the width of the hysteresis loop and power losses during magnetization reversal.
Magnetization curve. The process of magnetization of a ferromagnetic material can be depicted in the form of a magnetization curve (Fig. 44, a), which represents the dependence of the induction B on the magnetic field strength H. Since the magnetic field strength is determined by the strength of the current through which the ferromagnetic material is magnetized, this curve can be considered as a dependence of induction on the magnetizing current I.
From nuclear energy to fishing pleasures
Lead is widely used in industry and everyday life.
Areas of application of lead
In medicine, X-ray studies are indispensable without metal covers.
In geology, a “heavy” metal is used to determine the age of the Earth and in particular its minerals and rocks. These are geochronological methods using isotopes of various elements, including lead.
We recommend: LITHIUM - in space, on earth, under water
If all lead-acid batteries disappeared tomorrow, we would have to switch to horses, bicycles or walk. Lead powers every car on the planet.
He, our gray hero, is included in soldering compounds. And try to prove to the fisherman that he can somehow manage without a lead sinker.
How metal became a murder weapon
It seems that humanity cannot live without wars, fights, showdowns, or at least hunting. The hobby of the strong half of humanity has always meant close contact with lead. Lead is easy to process. Even in ancient Rome, sling shells were cast from lead. It was later used to make bullets for centuries. To this day, bullets are made from lead for one simple reason - great penetration relative to the size and speed of the bullet.
In the first firearms, bullets were simply lead balls; now they are perfectly balanced little killers of different designs. But whether it is ordinary or special (armor-piercing, expansive, explosive), the bullet contains lead. Bullet cores are made of lead wire (for 45 caliber the wire is thicker, for 20 caliber it will be thinner). The cores are “dressed” in copper sleeves.
Lead nitrates, perchlorates and azides are used in the preparation of explosives.
Metal's dark past
Pliny and the physician Dioscorides wrote about the toxic properties of metal (they were concerned about the lead water pipes and metal tiles that lined the walls and sometimes the floor).
Russian Tsar Alexei Mikhailovich, while reconstructing the Kremlin, ordered the installation of a machine on the Vodovzvodnaya Tower that raised water into the Kremlin. Everything was fine, except the pipes were lead. Some historians and doctors blame the impact of the metal on the king’s offspring. His children, Fedor and Ivan, were very sick. Drinking lead water also affected Peter the Great. In the movies, the Tsar Father was handsome, but in life he was awkward, with narrow shoulders, a wide pelvis, long arms and legs.
Poisoning with toxic metal compounds continued further.
TPP (tetraethyl lead)
Our love for four wheels and speed led to the development of this substance. More precisely, to its addition to gasoline, which began to be called “leaded.” The cars ran faster on this kind of gasoline, and there were more and more of them. Things were heading towards a geochemical catastrophe - the thermal power plant was in the ground, in the air, in human bodies. The governments of the countries realized that at this rate, with lead poisoning, there will soon be no one to rule. And they introduced a ban on leaded gasoline.
Related publications
- PLATINUM - “rotten silver” or noble metal
- BRONZE - an alloy for all times and peoples
- BISMUT - radioactive and safe
- TANTALUM - hard, rare and expensive
Lead ore deposits
Lead is formed in hydrothermal deposits, in zones of oxidation of polymetallic ores, in black clays and shales.
Found in nature mainly in the form of compounds and minerals. The main mineral for metal mining is galena, PbS, lead luster.
Galena, Dalnegorsk skarn deposit
Pliny in Natural History reports on the extraction of metal:
“We use black lead for pipes and slabs; it is dug with difficulty in Spain and all Gaul, but in Britain it is obtained from the upper layer of the earth in such abundance that a law has been passed limiting the extraction to a certain extent.”
Black lead was then called lead itself, white or silver - tin. Well, there were no educated mineralogists then...
What metals are not magnetic: list
There are only 9 ferromagnets, that is, metals that are highly magnetic, in nature. These are iron, cobalt, nickel, their alloys and compounds, as well as six lanthanide metals: gadolinium, terbium, dysprosium, holmium, erbium and thulium.
Metals that are attracted only to very strong magnets (paramagnetic): aluminum, copper, platinum, uranium.
Since in everyday life there are no such large magnets that would attract a paramagnetic material, and also no lanthanide metals are found, we can safely say that all metals except iron, cobalt, nickel and their alloys will not be attracted to magnets.
So, what metals are not magnetic to a magnet:
- paramagnetic materials: aluminum, platinum, chromium, magnesium, tungsten;
- diamagnetic materials: copper, gold, silver, zinc, mercury, cadmium, zirconium.
In general, we can say that ferrous metals are attracted to a magnet, non-ferrous metals are not.
If we talk about alloys, then iron alloys are magnetic. These primarily include steel and cast iron. Precious coins can also be attracted to a magnet, since they are not made of pure non-ferrous metal, but of an alloy that may contain a small amount of ferromagnetic material. But jewelry made of pure non-ferrous metal will not be attracted to a magnet.
What metals do not rust and are not magnetic? These are ordinary food grade stainless steel, gold and silver items.