Aluminum products are used in all spheres of human activity. The sought-after metal has a lot of valuable advantages, but anodizing aluminum allows you to make it even better. We will understand how anodized aluminum is produced, what advantages it acquires, and what types of technology exist.
Aluminum sheet with anode coating Source tildacdn.com
Principles of the anodizing process
The process of electrochemical oxidation of aluminum and its alloys in solutions of sulfuric, chromic, oxalic acids and their mixtures is called aluminum anodization. Despite its apparent simplicity, the anodizing process has many options that directly affect the characteristics and quality of the oxide film. The appearance and structure of the coating is also affected by the composition of the aluminum alloy, and adjusting the electrolyte allows you to change the properties of the coating within a wide range. The quality and presence of impurities in the electrolyte composition can also be critical.
Anodizing differs significantly from the processes of electroplating metals (electrochemical deposition), in which a protective or decorative layer of metal is applied to the surface of a metal product, as it is a process of transforming the base metal, as a result of which the appearance and characteristics of the surface change.
Possibility of using anodized aluminum
Anodized parts are used in a wide variety of applications. This method is used to process interior items, dishes, handrails and other products that are used every day. This process is also used for suspended aluminum facades - they acquire increased resistance to external atmospheric influences.
Anodizing is used to protect parts of various equipment from corrosion. These are components for cars, airplanes, ships, and all kinds of aircraft. The treatment increases strength and provides increased resistance to stress.
Application of Anodizing
The use of anodization is a topic for a separate article; in any industry where products made of aluminum or its alloys are used to one degree or another and any properties of the metal need to be changed, anodization is the optimal and often the only solution.
Here is a list of the main areas of application of anodizing:
- Thin oxide films are used as a basis for applying organic and inorganic coatings (paint or varnish).
- Color anodizing. The use of various coloring electrolytes makes it possible to obtain a wide range of shades and colors of the surface of an aluminum product. Nickel, cobalt or tin salts are used as additives. The resulting shades range from light bronze to black.
- Increased wear resistance. Oxide coatings on aluminum are much harder than the base metal. Hard anodizing is widely used for parts subject to abrasion under light loads, as well as to increase the corrosion resistance of products.
- Electrical insulation. Compared to organic insulating materials, oxide film not only has high insulating properties, but also has significantly greater heat resistance.
- Obtaining a compacted surface with high anti-friction properties. (lubricating coating).
Briefly about the main thing
Anodizing aluminum improves and expands the performance characteristics of the metal. The essence of the process is the growth of an oxide film, the properties of which depend on the method of its preparation. In industrial conditions, hard anodizing is used, the oxide coating is durable and wear-resistant.
Warm anodizing allows you to obtain a not very strong porous structure, which, however, has good adhesion and can be painted with high quality. The result of the cold method is a thick layer of oxide with high anti-corrosion properties.
Selecting anodizing electrolyte
As mentioned above, the properties of the oxide film obtained by anodization are influenced by many factors - the type of aluminum alloy, the method of pre-treatment of the surface of the part, the anodizing mode and the type of finishing operations. The composition of the electrolyte is also decisive. Acid electrolytes are mainly used (alkaline electrolytes can be used in some cases for special types of anodizing). The main acid is sulfuric; the vast majority of anodizing electrolytes are prepared on its basis. Other acids are used to obtain special types of coatings.
Interference coloring of aluminum
Additional bath
Interference staining is a type of electrolytic staining. This method produces a wide range of colors due to the effect of optical interference. Typically, between anodizing and electrolytic painting, an additional operation (bath) is required to treat the anodic coating to expand the bottom of the pores to increase color intensity.
Demand is limited
The amount of metal deposited in conventional electroplating is greater than in standard interference coating. However, in the latter case, this metal is compactly “packed” at the bottom of the pores. The interference effect occurs between two light-scattering layers: the electro-chemically deposited metal layer at the bottom of the pores and the interface between the oxide layer and the aluminum located directly behind it.
Of all the colors obtained by this method, the gray-blue coating is considered the most attractive. This color dyeing method is not yet in widespread demand due to more complex technology and a limited range of colors.
Anodizing in sulfuric acid electrolyte
Anodizing in sulfuric acid makes it possible to obtain translucent, colorless coatings with a thickness of about 35 microns. If the anodizing process is preceded by a process of glossing the surface of the parts, the coatings obtain high decorative qualities (shiny anodizing). In sulfuric acid, plastic anodic films are also obtained, which are not destroyed when molding products.
Sulfuric acid concentration and electrolyte temperature
The concentration of sulfuric acid for anodizing in industrial conditions is taken in the range of 8-35% (by weight). In a concentrated solution, the anodic film becomes soft and porous, and the elasticity of the film is high. The classic concentration is 15% (by weight). The temperature during the anodizing process is set in the range from 180C to 250C. In most cases, a temperature of 200C is accepted. Using sulfuric acid, solid anodic films are also obtained; in this case, the anodizing process is carried out at low temperatures (from -5 to +5 0C).
Temperature control during the anodizing process is mandatory; the current density and the rate of film dissolution depend on the temperature, which in turn has a direct impact on the quality and characteristics of the coating. In order to avoid local overheating of the electrolyte solution, special mixing devices are used.
Voltage and current density
When anodizing in sulfuric acid, a standard rectifier with an output voltage of up to 24 volts is used. In standard mode, the current is 16 volts with a current density of 1.5 A/dm2. To obtain corrosion-resistant films of large thickness, the voltage and current are raised to 18 volts, and when processing aluminum-silicon alloys to 22 volts. In some cases, for example, when anodizing rolled material or wire, alternating current is used. The use of a reduced current density makes it possible to obtain thin, transparent oxide films that are superior in transparency to films of similar thickness obtained at standard current densities.
Process duration
The duration of the anodizing process depends on the required film thicknesses, as well as the current density used. For pure aluminum this ratio can be proposed as:
Film thickness, microns. = (Current density, a/dm2 X Time, min.)/3
The ratio is approximate, since the duration of the process may depend on the type of alloy and processing mode.
The working process
The technological process of anodizing differs from the processes of applying galvanic coatings primarily in that the dissipative ability of anodizing electrolytes is significantly higher than that of electrolytes used in the processes of chrome plating, copper plating, galvanizing or nickel plating of metal. Effective dispersive ability with active mixing makes it possible to obtain films of uniform thickness over the entire surface of the products, including the internal surfaces of holes and grooves.
Otherwise, the technological process of anodizing is similar to the processes of electrochemical coating - products are immersed in a preheated electrolyte on hangers or clamps, the parts do not come into contact with each other, the distance to the cathode must be at least 15 cm (for larger products, the values are higher). Then the solution is stirred and current is applied. Under normal conditions, the cathode area should be equal to the anode area, and the cathode cross-section should be sufficient to ensure the required current density.
At the end of the process, stop the current supply and immediately remove the products from the galvanic bath. The products are washed in running water and dried.
Technology
Electrochemistry
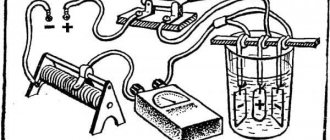
Aluminum anodization refers to the electrochemical processes of forming stable oxide coatings (films) on the surface of metals. Anodizing of aluminum and aluminum alloys can occur with the participation of a variety of electrolytes using direct or alternating current sources or combinations thereof. In this case, the aluminum product (hereinafter referred to as the profile for clarity) is always the anode, that is, it is connected to the positive pole of the current source, and another suitable metal or alloy is the cathode and is connected to the negative pole (Figure 1).
Anodic coatings are distinguished by the types of electrolytes that are used in their preparation. Coatings can be porous, for example, in phosphoric and sulfuric acid electrolytes, as well as so-called “barrier” - completely without pores. Barrier anodic coatings have high electrical resistance and are used, for example, in the manufacture of electrical capacitors.
Sulfate anodization
The usual, most popular and widely used for aluminum profiles in building structures is aluminum sulfate anodizing. This type of anodizing is highly technological and allows you to obtain coatings in a wide range of thicknesses. Sulfuric acid anodic coating is used both without additional coloring - it is called colorless, and with subsequent coloring using one of several well-known methods - it is called color anodizing. The final operation is usually always the operation of filling (or compacting) the pores.
Anodizing or painting aluminum
The sulfuric acid anodic coating is formed as a result of the “reaction” of aluminum with ions of a sulfuric acid solution. It occupies a larger volume than the original aluminum and therefore, as a result of anodization, the thickness of the product increases. With sulfuric acid anodizing, this increase is approximately one third of the total coating thickness. This is the fundamental difference between an anodic coating and, for example, a powder coating (Figure 2):
- the anodic coating is formed from a surface layer of aluminum,
- powder coating – on the surface of aluminum.
Figure 2 – Change in product thickness during anodizing and powder coating
Aluminum anodizing methods
The specific anodizing method depends on the type of product. For example, small products or parts can be anodized in bulk in drums or baskets. Profiles up to 7 m long, sometimes up to 10 m, are anodized on special hangers. These hangers usually consist of several conductive rods, frames or frames, to which profiles are firmly and fairly rigidly attached (see Figure 1). Strong fastening of the profiles is necessary so that they do not fall off the hangers and go through all the “dipping” and “rinsing” cycles in the baths, including during intensive mixing of solutions and rinsing waters (bubbling) / In addition, what is even more important, strong fastening of products to the hangers should ensure constant and reliable electrical contact of the profiles with the positive pole of the current source directly during the anodizing process.
Aluminum surface preparation
A typical anodizing line for aluminum profiles is shown in Figure 3.
Aluminum profiles are supplied to the anodizing line either directly after pressing, or after preliminary mechanical preparation of the surface (steel brushing, shot blasting, polishing, grinding, etc.).
- The first step in the anodizing process is to hang the profiles on hangers. A sample of aluminum profiles usually first undergoes alkaline degreasing and then alkaline etching to remove various contaminants from the surface of the profiles: oils, solid particles and oxide film.
- After alkaline etching, the sample is treated in a desmutting bath, most often sulfuric acid (80-100 g/l), to remove dark alkaline etching products from the surface.
- Treatment in baths with working solutions is accompanied by thorough rinsing of the products in water, the last rinsing before anodizing is in demineralized water. After this, the product is, in principle, ready for anodizing.
Figure 3 – Typical line of baths for anodizing aluminum profiles [1]
Matt anodizing
If there are special requirements for the anodized surface, additional surface treatment of the profiles is carried out: matte etching, as well as chemical or electrochemical brightening. Matt etching is usually carried out in alkaline baths of a special chemical composition. In this case, the surface layer of aluminum of a given thickness is removed along with various surface defects, and the surface becomes matte (Figure 4).
Figure 4 - Matte and shiny surface of anodized aluminum [3]
The matte surface scatters light as much as possible and makes remaining surface defects “invisible”. If the finished product must have a shiny or mirror surface, then before anodizing the product is subjected to chemical or electrochemical brightening. This procedure removes aluminum from the surface of the product and creates a very smooth surface with very high reflectivity.
Anodic coating filling
After anodizing, the profiles are either sent further down the line for painting, or immediately sent to fill the pores if it is colorless anodizing. The filling (or compaction) operation after clear anodizing or color anodizing is carried out in order to “close” or “clog” the pores of the anodic coating. This operation is very important to ensure long-term preservation of the appearance of the anodized product. After the filling operation, the products are dried, if necessary, removed from the hangers and sent for acceptance and packaging.
Figure 5 – Hydrothermal filling of the anodic coating [2]
Anodizing in chromic acid
Chromic acid is used if it is necessary to anodize critical aluminum parts and assemblies with thin walls or with high processing precision. The dissolution of aluminum in chromic acid is lower than in sulfuric acid, the reduction in the fatigue strength of the metal is lower - the film turns out to be thin, opaque gray in color. The maximum thickness of the oxide film reaches 10 microns, the standard thickness is from 2.5 to 5 microns.
The concentration of chromic anhydride CrO3 is taken in the range from 2 to 15% (by weight). In most cases, the regime temperature is set within the range of 25-400C; active stirring of the electrolyte solution is not required. When anodizing in a 10% solution of chromic acid, the process temperature is raised to 540C at a voltage of 30 volts to ensure a current density of 1.2 A/dm2. For alloys containing copper or zinc, the voltage is set within 15-20 volts at the same current density. When anodizing in an electrolyte of low concentration 3-5% (by mass), a special voltage supply mode is used and the process proceeds in cycles. This mode is used to detect defects in the surface of a product or when forming a sublayer for painting.
Anodization in oxalic acid
In a solution of oxalic acid, yellow films with high wear resistance are obtained. This method is one of the first open methods for obtaining a colored coating. The wear resistance of the coating during abrasion is two times higher than when anodizing in sulfuric acid. In the process of anodization in oxalic acid, along with direct current with a voltage of 30-60 volts, alternating current modes are used. To obtain a uniform yellow or bronze tint, the solution is vigorously mixed. Otherwise, this process is no different from anodization in sulfuric acid. Various metals can be used as cathodes - iron, lead, stainless steel.
Environmental impact
Anodizing aluminum is one of the most environmentally friendly metal processing processes. The process uses only very small amounts of heavy metals, halogens or organic compounds. The dyeing process does not produce VOCs or heavy metals. The recyclability of anodized and painted aluminum is equivalent to standard aluminum in terms of environmental impact. The waste from the anodizing process is easily recycled to produce alum, baking powder, newsprint and cosmetics. The waste can also be used for fertilizer and in industrial wastewater treatment plants.
Find out prices for services
Leave a request to receive a quick cost estimate for free
the service you are interested in. Managers will answer any of your questions!
Other anodizing solutions
In some cases, electrolytes are used in which the aluminum oxide film does not dissolve - so-called barrier-type electrolytes . Using anodizing solutions containing boric acid, ammonium tartrate, and ammonium borate, coatings are obtained on parts used in electrical appliances (electrolytic capacitors). For example, when treated in a solution with ammonium borate, films are obtained with a breakdown voltage of 550 volts. Also, these types of electrolytes are used in anodizing aluminum deposited in a vacuum.
Aluminum parts, the processing of which involves the application of galvanic coating after anodizing, are processed in a solution containing 25-30% phosphoric acid. The resulting films have a thickness of up to 6 microns, which is due to the high solubility of aluminum in phosphoric acid. The process is carried out at workshop temperature, current density 10-20 A/mm2 and voltage 30-60 volts for 10-15 minutes.
Solid films of golden, brown or black colors are obtained by using a solution containing 40-100 g/l sulfosalicylic acid and 30-60 g/l sulfuric acid at a temperature of 300C, a current density of 2.5-3.5 a/dm2 and a voltage of up to 80 volts.
Adsorption coloring of aluminum
Hundreds of dyes
This method is used for hundreds of different dyes. An aluminum product with a colorless anodic coating, not yet filled, is immersed in an aqueous (rarely alcohol) solution, usually an organic dye. The intensity of the color depends on the amount of dye absorbed by the anodic coating. The dye is absorbed only 3-4 microns deep into the pores of the anodic coating. The coating is then compacted.
Technology
For good coloring, as well as high corrosion resistance, an anodic layer thickness of at least 15 microns is required. The concentration of dye solutions can range from 0.2-0.4 g/l for light colors, to 10 g/l for rich tones. Typically, hot dye solutions are used - from 55 to 75 ºС, and the dyeing duration is from 5 to 15 minutes; saturated colors may require 30 minutes. An important parameter for dye adsorption is the pH of the solution, the optimal range is usually from 5 to 6.
Removing anodic coatings
Poor-quality anodic coating can only be removed from the entire surface of the product; partial restoration of the film is impossible in most cases. The coating is usually removed in solutions containing caustic alkalis. The process takes place under strict control of the main modes, since such solutions have a high degree of impact on the base metal. The classic solution that has the least impact on the aluminum surface is one containing 35 ml/l phosphoric acid and 20 g/ml chromic acid. Treatment takes place within 1-10 minutes, depending on the thickness of the film at a temperature of 95-1000C. to remove hard anodic coatings, use the specified solution with a concentration twice as high, while the surface of aluminum alloys containing copper can be painted gray or black.
Re-processing of products after removing the anodic film is possible after assessing the condition of the surface of the product; if the surface cleanliness is sufficient for coating and polishing is not required, you can begin the process immediately.
It should be noted that when processing parts for which it is necessary to strictly adhere to the original dimensions, repeated anodization will be required with the application of a film of greater thickness than it was originally. This is because when the coating is removed and reapplied, losses can range from half to two-thirds of the original film thickness.
You may be interested in the following articles:
|
Methods for color anodizing aluminum
Aluminum and aluminum alloys can be painted in different tones and colors, both during the anodizing process itself and after it. Common, most popular methods for painting anodized aluminum profiles include (Figure 1):
- adsorption coloring;
- electrolytic dyeing;
- integral staining;
- interference staining.
Figure 1 – Methods for painting an anodic coating