Pure copper is not used for the manufacture of products. It is used as ready-made alloys, the compositions of which are regulated by generally accepted standards. In Russia, the main regulation is GOST 859-2001. He describes in detail the grades and compositions of copper alloys, as well as the permissible areas of their use.
Since copper is a non-ferrous metal with unique physical and chemical properties, it is actively used in industry, production, construction and domestic conditions. There is also a separate classification of copper scrap, which is purchased on the secondary market.
Technical copper
In the annealed state, it is quite plastic, but has relatively low strength. The chemical composition of technical grades of copper is determined by GOST 859–41. Technical grades are used in the smelting of copper alloys as a charge material. Technical grade M0 is used in the manufacture of high-purity alloys and current conductors. M1 in the manufacture of semi-finished products obtained by rolling, in the production of high-quality bronzes that do not contain tin. M2 is a technical grade used for the production of bronzes for the production of high-quality semi-finished products that are processed under pressure. The MZ technical grade is in demand for semi-finished products that are produced by rolling, the production of standard quality bronzes and other casting alloys, as well as non-critical electrical contacts (like the M2 alloy). Technical grade M4 is used in the smelting of cast bronzes.
Pipe manufacturing
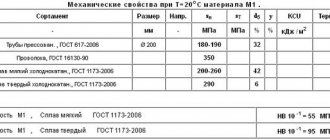
In order to produce high-quality copper pipes suitable for further use, you need to know which brand to use, as well as comply with certain technical requirements that are prescribed in GOST 617-90. Thus, in industrial production the M3 brand is used, as well as M1, M1r, M2, M2r, M3r, GOST 859 and chemical. composition GOST 15527 L96.
Pipes come in the following varieties: pressed and cold-deformed, hard, medium-hard and soft.
Impurities in copper alloys
Impurities contained in copper (and, naturally, interacting with it) are divided into three groups.
Forming solid solutions with copper
Such impurities include aluminum, antimony, nickel, iron, tin, zinc, etc. These additives significantly reduce electrical and thermal conductivity. The grades that are primarily used for the production of conductive elements include M0 and M1. If the copper alloy contains antimony, its hot pressure treatment becomes significantly more difficult.
Impurities that do not dissolve in copper
These include lead, bismuth, etc. Although they do not affect the electrical conductivity of the base metal, such impurities make it difficult to process by pressure.
Impurities that form brittle chemical compounds with copper
This group includes sulfur and oxygen, which reduces the electrical conductivity and strength of the base metal. The sulfur content of the copper alloy greatly facilitates its machinability by cutting.
Copper grades and their applications
Copper rods. Conventions. GOST 1535-2006.
The symbols of copper rods used in documentation and certificates are standardized according to GOST 1535-2006, “Copper rods”.
Recording a symbol
| Bar | X | XX | X | X | … | … | … | … | GOST 1535-2006 |
| Product type | |||||||||
| Preparation method | |||||||||
| Section shape | |||||||||
| Precision manufacturing | |||||||||
| State | |||||||||
| Section dimensions | |||||||||
| Length | |||||||||
| Copper grade | |||||||||
| Special conditions | |||||||||
| Standard | |||||||||
The following abbreviations are used:
- KR - round
- KV - square
- SHG - hexagonal
- N - normal
- P - increased
- B - high
- M - soft
- P - semi-solid
- T - hard
- A - permissible diameter deviations with symmetrical tolerances
- AB - for processing on automatic machines
- L - soft state of increased plasticity
- F - semi-solid state of increased plasticity
- Y - solid state of increased plasticity
- OK - with cut ends
- C - increased accuracy in curvature
- P - regulated requirements for tensile testing
- HB - regulated requirements for Brinell hardness testing
- HV - regulated requirements for Vickers hardness measurement
- BT - rod in free-winding coils
- BU - rod in coils of layer-by-layer ordered winding
An “X” is placed in place of missing data, except for the length designation and special conditions.
If special conditions are not specified by the consumer in the order, then the rods are manufactured with performance conditions at the discretion of the manufacturer.
Examples of bar symbols
Drawn rod, round, high-precision manufacturing, hard, 10 mm in diameter, of unmeasured length, made of M1 grade copper, intended for processing on automatic machines:
- Rod DKRVT 10 ND M1 AB GOST 1535-2006
Drawn rod, hexagonal, high-precision manufacturing, soft, diameter 19mm, length 3000mm, made of copper grade M2:
- Rod DShGPM 19 x 3000 M2 GOST 1535-2006
Drawn rod, square, of normal manufacturing precision, semi-solid, with a diameter of 10 mm, a multiple of the measured length of 1500 mm, from copper grade MZ:
- Rod DKRNP 10 x 1500 KD MZ GOST 1535-2006
Drawn rod, round, high precision manufacturing, semi-solid, diameter 10mm. of unmeasured length, made of copper grade M1, increased ductility, with regulated tensile test requirements, intended for processing on automatic machines:
Pressed rod, round, diameter 35mm, unmeasured length, made of MZ copper:
- Rod GKRHH 35 ND MZ GOST 1535-2006
Percentage composition
| Percentage composition of material | |||||||||||||
| Copper alloy grades | Fe | Ni | S | Cu | As | Pb | O | Sb | Bi | Sn | P | Zn | Ag |
| M1 | ≤ 0.005 | ≤ 0.0020 | ≤ 0.004 | 99.9 | ≤ 0.0020 | ≤ 0.005 | ≤ 0.05 | ≤ 0.002 | ≤ 0.001 | ≤ 0.002 | ≤ 0.004 | ≤ 0.003 | |
| M 1р | ≤ 0.005 | ≤ 0.0020 | ≤ 0.005 | 99.9 | ≤ 0.0020 | ≤ 0.005 | ≤ 0.01 | ≤ 0.002 | ≤ 0.001 | ≤ 0.002 | from 0.002 to 0.012 | ≤ 0.005 | |
| M 2 | ≤ 0.05 | ≤ 0.2 | ≤ 0.01 | 99.7 | ≤ 0.01 | ≤ 0.01 | ≤ 0.07 | ≤ 0.005 | ≤ 0.0020 | ≤ 0.05 | |||
| M 2p | ≤ 0.05 | ≤ 0.2 | ≤ 0.01 | 99.7 | ≤ 0.01 | ≤ 0.01 | ≤ 0.01 | ≤ 0.005 | ≤ 0.0020 | ≤ 0.05 | |||
| M 3 | ≤ 0.05 | ≤ 0.2 | ≤ 0.01 | 99.5 | ≤ 0.01 | ≤ 0.050 | ≤ 0.08 | ≤ 0.050 | ≤ 0.003 | ≤ 0.05 | |||
| M 3р | ≤ 0.05 | ≤ 0.2 | ≤ 0.01 | 99.5 | ≤ 0.05 | ≤ 0.03 | ≤ 0.01 | ≤ 0.05 | ≤ 0.003 | ≤ 0.05 | from 0.005 to 0.06 |
Use of copper in medicine
The use of copper in the medical industry can be found quite often. According to the norms of traditional medicine, copper is an extremely important element of human life. In our body, copper is present in a volume of 2 * 10-4% of a person’s total weight. Every day we consume approximately 60 mg of copper with food, but only 2 mg is absorbed, but this is the amount that is the daily norm for an adult. Copper is extremely important in the process of hemoglobin biosynthesis, as well as in maintaining sugar, cholesterol and uric acid levels. In order for the cardiovascular system, brain, and digestive tract to function as expected, copper is needed. With a chronic lack of copper in the human body, the following diseases develop:
- anemia;
- osteoporosis;
- glaucoma;
- psoriasis;
- the heart muscle atrophies;
- a person gets tired quickly and loses weight;
- Cholesterol accumulates in the body.
The richest foods containing copper are:
- Champignon;
- potato;
- Cod liver;
- whole grain;
- oysters and cuttlefish.
Pure copper
Grade M0 contains 99.95% Cu and no more than 0.05% impurities. According to special technical conditions, several grades of vacuum copper and especially oxygen-free pure copper are produced, which is used in the electric vacuum industry. Strips, tapes, rods, and pipes are produced from oxygen-free copper of series A and B. Tapes and rods are made from vacuum pure copper. Rods are produced from pure copper, which is deoxidized with manganese. All these semi-finished products are used in the electrovacuum industry. Oxygen-free pure copper is characterized by a low (-100°C) recrystallization temperature.
Production of strips and sheets
Strips and sheets are made in accordance with GOST 495-92, for this they use copper with the following markings: M1, M1r, M2, M2r, M3, M3r GOST 859.
Normal and high precision production methods are used for cold-rolled sheets and strips.
The size of hot-rolled sheets varies from six hundred to three thousand mm in width, and in length - from one thousand to six thousand.
According to the degree of hardness, cold-rolled sheets and strips on an industrial scale are soft, hard, and medium.
Basic properties of copper
Physical properties
In air, copper acquires a bright yellowish-red hue due to the formation of an oxide film. Thin plates have a greenish-blue color when examined through them. In its pure form, copper is quite soft, malleable and easily rolled and drawn. Impurities can increase its hardness.
This is interesting: Ejector - what is it? The principle of operation of ejector pumps and their design
The high electrical conductivity of copper can be called the main property that determines its predominant use. Copper also has very high thermal conductivity. Impurities such as iron, phosphorus, tin, antimony and arsenic affect the basic properties and reduce electrical and thermal conductivity. According to these indicators, copper is second only to silver.
Copper has high densities, melting points and boiling points. An important property is also good resistance to corrosion. For example, at high humidity, iron oxidizes much faster.
Copper lends itself well to processing: it is rolled into copper sheets and copper rods, and drawn into copper wire with a thickness brought to thousandths of a millimeter. This metal is diamagnetic, that is, it is magnetized against the direction of the external magnetic field.
Chemical properties
Copper is a relatively low-active metal. Under normal conditions in dry air, its oxidation does not occur. It reacts easily with halogens, selenium and sulfur. Acids without oxidizing properties have no effect on copper. There are no chemical reactions with hydrogen, carbon and nitrogen. In humid air, oxidation occurs to form copper (II) carbonate, the top layer of platinum. Copper is amphoteric, meaning it forms cations and anions in the earth's crust. Depending on the conditions, copper compounds exhibit acidic or basic properties.
Supply
Are you interested in technical and pure copper? The supplier "Auremo" offers to buy technical and pure copper today on favorable terms. Large selection in stock. Full compliance with GOST and international quality standards, optimal price from the supplier. We offer to buy technical and pure copper from specialized warehouses with delivery to any city. Buy today. For wholesale customers the price is preferential.
Buy, favorable price
Technical and pure copper from the supplier “Auremo” complies with GOST and international quality standards, the price is optimal. We have the widest selection of products in stock. Technical and pure copper is always available, the price is determined by the technological features of production without including additional costs. Optimal price from the supplier. Buy today. We are waiting for your orders. We have the best price-quality ratio for the entire range of products. Experienced managers are in touch and will quickly help you buy rolled copper wholesale or in installments. Regular customers can buy rolled copper at a discount.
Features of popular copper alloys
Alloy M1 is manufactured in accordance with GOST 859-2014, is a highly plastic and well-processed metal, and has the highest copper content (99.9%). Additional elements include zinc, nickel, phosphorus, iron, arsenic, oxygen, tin, bismuth (total no more than 0.1%). The electrical resistivity is 0.018 μOhm. The alloy can be of two types - hard (M1t) and soft (M1m), they differ in strength and fluidity. Rolled metal is in demand in the automotive and aircraft industries, in the creation of current conductors, cryogenic equipment, wire and rods.
Alloy M2 has a lower copper ratio in the composition (99.7%). The remaining 0.3% comes from nickel, iron, antimony, oxygen, tin, lead, sulfur, and arsenic. This grade is ductile and does not rust, is excellent in pressure processing and is used for the manufacture of copper-based alloys and refrigeration parts.
Alloy M3 is technical copper; it contains the smallest percentage of metal among those presented (99.5%). The same elements as in M2 are used as alloying components, only in a larger proportion (up to 0.5%), which makes this alloy the most affordable. Optimally suitable for metal products that are sold by rolling methods, as well as cast alloys.
Copper m1 – Copper grades according to GOST 859 M1, M2, M3
alexxlab | 12/05/2018 | 0 | Questions and answers
- Copper grades according to GOST 859 M1, M2, M3
- Copper M1 / Auremo
- Copper M1r / Auremo
- properties, characteristics, composition.
Mass fraction of impurities in copper M1 GOST 859-2001 - Copper grades - classification, physical properties, application +
- MV copper (vacuum copper) + Anodes, graphite, solder... › Russian metal We offer MV copper to order.
- We supply electrical copper M1E to order.
Copper grades according to GOST 859 M1, M2, M3
Due to their properties, various grades of copper are very popular in the industrial environment. This metal is good because it is flexible and, regardless of the operating environment, with the exception of exposure to sulfur dioxide and ammonia, it is resistant to corrosion.
The external distinctive feature of copper is its pink-red color. Depending on the purity, copper is divided into types with the technical designations M1, M2, M3. This metal comes into production in the form of wire, sheets, pipes, and rods.
This is due to different application situations.
Schedule
Based on its composition, copper is divided into oxygen-free and deoxidized; the symbol is M0 and M1, respectively. Oxygen-free is used in the manufacture of parts for electrical, electronic, and electrovacuum industrial products. O2 in oxygen-free brands is no more than 0.001%, and in deoxidized ones - 0.01%.
The breakdown of copper grades is presented in the table:
Designations
NameMeaning
| Designation GOST Cyrillic | M1 |
| Designation GOST Latin | M1 |
| Translit | M1 |
| By chemical elements | Cu1 |
Description
M1 copper is used : for the production of current conductors; rental; high-quality tin-free bronzes; cryogenic equipment products; round drawn thin-walled pipes; cold-rolled foil and strip, cold-rolled and hot-rolled sheets and plates for general purposes; wires for the manufacture of metal shielding braids of the PML type, intended for shielding wires and cables; hot-rolled and cold-rolled anodes used for galvanic coating of products; cold-deformed rectangular tape with a thickness of 0.16−0.30 mm, intended for coaxial trunk cables; radiator tapes intended for the manufacture of cooling tubes and radiator plates; drawn pipes of rectangular and square cross-section, intended for the manufacture of conductors for stator windings of liquid-cooled electrical machines; profiles for the manufacture of rotors of submersible electric motors; round welding wire and round welding rods drawn and pressed with a diameter from 1.2 to 8.0 mm, intended for automatic welding in an inert gas environment, submerged arc and gas welding of non-critical structures made of copper, as well as the manufacture of electrodes for welding copper and cast iron.
Note
M1 copper is obtained by melting cathodes. Copper grade M1 in chemical composition corresponds to copper grade Cu-ETP according to Euronorm EN 1652:1998.
Biological value for humans
Copper belongs to the category of vital elements, and the body of an adult contains about 100 grams of this metal. A reassessment of the toxicity of this substance was carried out in 2003 by the World Health Organization. Studies have found that copper is not a cause of diseases of the digestive tract, and does not provoke the development of Wilson-Konovalov disease (hepatocerebral dystrophy affecting the liver and brain), as previously thought. Scientists have concluded that a lack of copper is more harmful to human health than its excess.
The bactericidal properties of copper have been known for a long time, and recent studies in this area have confirmed the effectiveness of the metal in the prevention of swine flu and infection by Staphylococcus aureus. In experiments, it was found that 99% of pathogenic bacteria die on a copper surface within 2 hours. Therefore, copper and its alloys are widely used for water disinfection. In Europe, door handles, locks, hinges and railings are made from this metal, which are installed in medical institutions and public places.
Marking
In addition to differences in grades, there are different markings of this non-ferrous metal (according to its purity):
The last element of the marking is a letter that indicates the method of copper production (k - cathode, f - deoxidized with phosphorus, b - oxygen-free, p - deoxidized).
accepts various types of copper at our points, as well as at the client’s premises (in case of large volumes of scrap). We will determine the type of metal and, if necessary, send it for additional chemical examination. We work officially, so we provide a full package of documents for supervisory authorities.
Source
Methods for obtaining copper
In nature, copper exists in compounds and in the form of nuggets. The compounds are represented by oxides, bicarbonates, sulfur and carbon dioxide complexes, as well as sulfide ores. The most common ores are copper pyrite and copper luster. The copper content in them is 1-2%. 90% of primary copper is mined using the pyrometallurgical method and 10% using the hydrometallurgical method.
1. The pyrometallurgical method includes the following processes: enrichment and roasting, smelting for matte, purging in a converter, electrolytic refining. Copper ores are enriched by flotation and oxidative roasting. The essence of the flotation method is as follows: copper particles suspended in an aqueous medium adhere to the surface of air bubbles and rise to the surface. The method allows you to obtain copper powder concentrate, which contains 10-35% copper.
Copper ores and concentrates with a significant sulfur content are subject to oxidative roasting. When heated in the presence of oxygen, sulfides are oxidized, and the amount of sulfur is reduced by almost half. Poor concentrates containing 8-25% copper are roasted. Rich concentrates containing 25-35% copper are melted without resorting to roasting.
The next stage of the pyrometallurgical method for producing copper is smelting for matte. If lump copper ore with a large amount of sulfur is used as a raw material, then smelting is carried out in shaft furnaces. And for powdered flotation concentrate, reverberatory furnaces are used. Melting occurs at a temperature of 1450 °C.
In horizontal converters with side blowing, the copper matte is blown with compressed air in order for the oxidation of sulfides and ferrum to occur. Next, the resulting oxides are converted into slag, and sulfur into oxide. The converter produces blister copper, which contains 98.4-99.4% copper, iron, sulfur, as well as small amounts of nickel, tin, silver and gold.
This is interesting: Why are metallic sounds heard in the car after turning off the engine?
Blister copper is subject to fire and then electrolytic refining. Impurities are removed with gases and converted into slag. As a result of fire refining, copper is formed with a purity of up to 99.5%. And after electrolytic refining, the purity is 99.95%.
2. The hydrometallurgical method involves leaching copper with a weak solution of sulfuric acid, and then separating copper metal directly from the solution. This method is used for processing low-grade ores and does not allow for the associated extraction of precious metals along with copper.
Reserves, production
The world's proven reserves of copper ore amount to billions of tons. Scientists hope that the earth's bowels conceal another 3 billion tons. The richest mines, Chuquicamata and El Teniente, are located in Chile, the largest copper producer (27% of world production in 2022).
Large deposits have been found in the USA, Canada, Iran, Kazakhstan, and southern Africa. Russia contains 3% of the world's reserves. Copper deposits have been found in the Urals, Transbaikalia, and Krasnoyarsk Territory.
It is desirable that the ore contains at least 0.5-1% pure copper. It is mined in mines and by open-pit mining (for deposits located at a depth of 400-500 m). Global production in 2022 amounted to 20.6 million tons, of which a quarter was of Chilean origin.
Copper alloys, their properties, characteristics, grades
The production of copper alloys makes it possible to improve the properties of copper without losing the main advantages of this metal, as well as to obtain additional useful properties.
Copper alloys include: bronze, brass and copper-nickel alloys.
Brass
This is an alloy of copper and zinc. In addition to zinc, it also contains other alloying additives, including tin.
Brasses are corrosion-resistant alloys. They have anti-friction properties to resist vibrations. They have high fluidity rates, which gives products made from them a high degree of resistance to heavy loads. In brass castings, segregation areas are practically not formed, so the products have a uniform structure and density.
Brasses are marked with a set of alphanumeric codes, where the first letter is always L, meaning brass itself. Next comes a digital indicator of the percentage of copper in brass. The remaining letters and numbers indicate the content of alloying elements as a percentage. Brasses use the same letter designations for alloying elements as bronzes.
An example of double brass marking: L85. It stands for “brass with a copper content of up to 85%, the rest is zinc.”
An example of multi-component brass marking: LMtsA57-3-1. It stands for “brass with a copper content of up to 57%, manganese - up to 3%, aluminum - up to 1%, the rest is zinc.”
Bronze
An alloy of copper and tin. However, with the development of technology, bronzes also appeared, in which, instead of tin, aluminum, silicon, beryllium and lead were introduced into the alloy.
Bronze is harder than copper. They have higher strength ratings. They are better suited to metal forming, especially forging.
Marking of bronzes is carried out using alphanumeric codes, where the first letters are Br, meaning bronze itself. Additional letters indicate alloying elements, and numbers after the letters indicate the percentage of such elements in the alloy.
Letter designations of bronze alloying elements:
- A – aluminum,
- B – beryllium,
- F – iron,
- K – silicon,
- Mts – manganese,
- N – nickel,
- O - tin,
- C – lead,
- C – zinc,
- F – phosphorus.
An example of marking tin bronze: BrO10S12N3. It stands for “tin bronze with a tin content of up to 10%, lead – up to 12%, nickel – up to 3%.”
An example of decoding aluminum bronze: BrAZh9-4. It stands for “aluminum bronze with an aluminum content of up to 9% and iron up to 4%.”
Copper-nickel alloys
- Cupronickel is an alloy of copper and nickel. Iron and manganese may be present in the alloy as additives. Special cases of technical alloys based on copper and nickel:
- Nickel silver – additionally contains zinc,
- Constantan – additionally contains manganese.
Cupronickel has high corrosion resistance. It lends itself well to any type of mechanical processing. Non-magnetic. It has a pleasant silver color.
Due to its properties, cupronickel is, first of all, a decorative and applied material. Jewelry and souvenirs are made from it. For decorative purposes it is an excellent substitute for silver.
There are 2 brands of cupronickel available:
- MNZHMts – an alloy of copper with nickel, iron and manganese;
- MH19 is an alloy of copper and nickel.
Stainless steel devices
stainless steel apparatus As a rule, in the correct production of moonshine units, steel is used, which complies with the standards for materials and products in contact with food.
In reviews of distillers from experienced moonshiners, in most cases there are no complaints about the quality of stainless steel materials when used in moonshine. This material has a number of undeniable advantages: it is resistant to corrosion, does not require additional maintenance, and has excellent thermal conductivity. This material has a relatively reasonable cost, and most models of moonshine stills are produced in factories on machines.
Source