| HOUR | He | |||||||||||||||||
| Lee | Be | B | C | N | ABOUT | F | Ne | |||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| K | Ca | Sc | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||
| Rub. | Sr | Y | Pd | Ag | CD | IN | Sn | Sb | Te | I | Xe | |||||||
| C.S. | Ba | La | * | OS | Pt | Au | Hg | Tl | Pb | Bi | By | IN | Rn | |||||
| Fri | Ra | Ac | ** | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | C | Og |
| * | Ce | Pr | Nd | Evenings | Cm | Europe | G-d | Tb | Dy | Ho | E | Tm | Yb | Lou | ||||
| ** | Thu | Pa | U | Np | Pu | I am | Cm | Bk | Cf | Es | FM | Microdistrict | No | Lr | ||||
| Refractory metals | ||||||||||||||||||
| Broader definition of refractory metals[1] | ||||||||||||||||||
Refractory metals
are a class of metals that are extremely resistant to heat and wear. The expression is mainly used in the context of materials science, metallurgy and engineering. The definition of which elements belong to this group varies. The most common definition includes five elements: two from the fifth period (niobium and molybdenum) and three from the sixth period (tantalum, tungsten, and rhenium). They all share common properties, including a melting point above 2000 °C and high temperature. hardness at room temperature. They are chemically inert and have a relatively high density. Their high melting points make powder metallurgy the method of choice for making components from these metals. Some of their applications include tools for processing metals at high temperatures, wire filaments, foundry molds, and vessels for chemical reactions in corrosive environments. Partly because of their high melting point, refractory metals are resistant to creep deformation up to very high temperatures.
Definition
Most definitions of the term "refractory metals" list an extremely high melting point as a key requirement for inclusion. One definition requires a melting point greater than 4,000 °F (2,200 °C) to qualify.[2] The five elements niobium, molybdenum, tantalum, tungsten and rhenium are included in all definitions,[3] while the broader definition, including all elements with a melting point above 2123 K (1850 °C), includes varying amounts of nine additional elements: titanium, vanadium, chromium, zirconium, hafnium, ruthenium, rhodium, osmium and iridium. The artificial elements, being radioactive, are never considered part of the refractory metals, although technetium has a melting point of 2430 K or 2157 °C and rutherford is predicted to have a melting point of 2400 K or 2100 °C.[4]
Answers@Mail.Ru: which metal is the most refractory?
Tungsten is certainly good. But among other classes of substances there are also more refractory ones. To date, a record melting temperature has been found for mixed hafnium-tantalum carbide, according to different versions from 3900 to 4200 degrees Celsius. Such a large error is due, firstly, to the fact that there are still no particularly accurate methods for measuring ultra-high temperatures, and secondly, it depends on the chemical purity of the material (foreign impurities reduce the melting point)
Platinum hmm... or iron
tungsten and platinum
tungsten. about 3000°C.
Tungsten. - 3410 degrees, then rhenium - 3180, tantalum - almost 3000
As far as I know, it's tungsten.
tungsten. melting point=3380gr. Celsius. The most refractory of metals. After all, the question is about METALS, and not about chemical compounds or alloys. Indeed, the alloy of hafnium carbide (HfC, 20%) and tantalum carbide (TaC, 80%) is the most refractory ALLOY (mp 4216 °C). In addition, there are individual indications that when alloying this alloy with a small amount of titanium carbide, the melting point can be increased by another 180 degrees.
If you do not take into account the chemical connections, then tungsten. It is used in light bulbs
And it is its filament that burns out when the light bulb becomes unusable
It is used in light bulbs. And it is its filament that burns out when the light bulb becomes unusable.
CALCIUM CARBITE MELTING POINT 4000
touch.otvet.mail.ru
Characteristics
Physical
Properties of refractory metals
| Name | Niobium | Molybdenum | Tantalum | Tungsten | Rhenium |
| Period | 5 | 5 | 6 | 6 | 6 |
| Melting point K | 2750 | 2896 | 3290 | 3695 | 3459 |
| Boiling point K | 5017 | 4912 | 5731 | 5828 | 5869 |
| Melting point °C | 2477 | 2623 | 3017 | 3422 | 3186 |
| Boiling point °C | 4744 | 4639 | 5458 | 5555 | 5596 |
| Density g cm−3 | 8.57 | 10.28 | 16.69 | 19.25 | 21.02 |
| Module for junior GPA | 105 | 329 | 186 | 411 | 463 |
| Vickers hardness MPa | 1320 | 1530 | 873 | 3430 | 2450 |
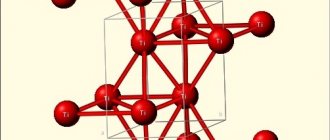
The melting point of refractory metals is highest for all elements except carbon, osmium and iridium. This high melting point determines most of their applications. All metals are body-centered cubic except rhenium, which is hexagonal close-packed. Most of the physical properties of the elements in this group vary significantly because they are members of different groups.[5][6]
Creep resistance is a key property of refractory metals. In metals, the onset of creep correlates with the melting point of the material; Creep in aluminum alloys begins at 200 °C, whereas refractory metals require temperatures above 1500 °C. This resistance to deformation at high temperatures makes refractory metals suitable for withstanding high impact forces at high temperatures, such as in jet engines, or tools, used during forging.[7][8]
Chemical
Refractory metals exhibit a wide range of chemical properties because they fall into three distinct groups on the periodic table. They oxidize easily, but in bulk metal this reaction is slowed down by the formation of stable oxide layers on the surface. In particular, rhenium oxide is more volatile than the metal, and therefore at high temperatures the stabilization against oxygen is lost as the oxide layer evaporates. All are relatively resistant to acids.[5]
Production of final products to order
The narrow specialization of the site, as well as the high cost of metals, does not allow us to have in stock the entire variety of products made from refractory alloys. Therefore, this site presents only the most popular types of products, which, as a rule, have standard parameters (technical characteristics, properties, sizes, shapes). This feature is well known to our regular clients, many of whom base their cooperation on individual interaction. They order the manufacture of specific products according to their own drawings, reflecting in the order not only the requirements for geometry, but also composition, properties, operating conditions, etc.
The use of refractory metals due to their poor machinability requires the use of special equipment. The group has the most modern production facilities, allowing:
- perform specific types of work (cut rods of the required length, drill holes of a specific size, etc.);
- restore/update old parts, tape;
- form a winding from refractory wire of the required diameter, density and length;
- manufacture any types of electrodes, standard products from rare grades of refractory steels, introduce unique developments;
- find optimal compositions, shapes and sizes to solve specific problems assigned to us;
- manufacture products with high geometric accuracy, which is especially important when working with professional machines, instruments, and equipment.
Our team consists of specialists from various fields, which allows us to solve the most complex problems in a short time. Why buy expensive equipment for working with hard alloys and refractory metals when you can entrust a number of works to a third party? In 90% of cases, this approach has good financial prospects, making cooperation with the group mutually beneficial and promising.
In this section of the site you can buy: polycor of different brands and sizes, tungsten waveguide, refractory targets, thermocouple wire, ready-made thermocouples, converters, screen units of furnaces, riveted heaters, molybdenum and tungsten screens, electrodes, evaporators, bent, straight, strip, plate heaters, vacuum ovens and much more. Questions about the availability of products and their selection can be asked to our managers.
Applications
Refractory metals are used in lighting, tools, lubricants, nuclear reaction control rods, catalysts, and for their chemical or electrical properties. Due to their high melting point, refractory metal components are never produced by casting. The powder metallurgy process is used. Pure metal powders are compacted, heated by electric current, and then produced by cold working and annealing. Refractory metals can be processed into wire, ingots, fittings, sheets or foil.
Molybdenum alloys
Main articles: Molybdenum and Molybdenum § Applications
Molybdenum-based alloys are widely used because they are less expensive than higher quality tungsten alloys. The most widely used molybdenum alloy is T
itanium-
Zirconium
- M-
olybdenum alloy TZM, consisting of 0.5% titanium and 0.08% zirconium (the rest is molybdenum). The alloy exhibits higher creep resistance and strength at high temperatures, allowing the material to be used at temperatures above 1060°C. The high resistance of Mo-30W, an alloy consisting of 70% molybdenum and 30% tungsten, against attack by molten zinc makes it an ideal material for zinc casting. It is also used to make zinc molten valves.[9]
Molybdenum is used in mercury contact reed relays because molybdenum does not form an amalgam and is therefore resistant to corrosion by Mercury fluid.[10][11]
Molybdenum is the most commonly used refractory metal. Its most important use is as a reinforcing alloy of steel. Structural pipes and piping often contain molybdenum, as do many stainless steels. Its strength at high temperatures, wear resistance and low coefficient of friction all these properties make it an invaluable alloying compound. Its excellent anti-friction properties lead to its inclusion in greases and oils where reliability and performance are critical. Automotive constant velocity joints use a lubricant containing molybdenum. The composition easily adheres to metal and forms a very hard, friction-resistant coating. Most of the world's molybdenum ore can be found in China, the United States, Chile and Canada.[12][13][14][15]
Tungsten and its alloys
Main articles: Tungsten and Tungsten § Applications
Tungsten was discovered in 1781 by the Swedish chemist, Karl Wilhelm Scheele. Tungsten has the highest melting point of all metals at 3,410°C (6,170°F).
200W high magnification incandescent lamp filament
Up to 22% of rhenium is alloyed with tungsten to increase its heat resistance and corrosion resistance. Thorium is used as an alloying compound when an electric arc occurs. Ignition is easier and the arc burns more consistently than without the addition of thorium. In powder metallurgy, binders must be used during the sintering process. To produce heavy tungsten alloy, binder mixtures of nickel and iron or nickel and copper are widely used. The tungsten content in the alloy usually exceeds 90%. The distribution of binding elements into tungsten grains is small even at sintering temperatures and, therefore, the interior of the grains is pure tungsten.[16]
Tungsten and its alloys are often used in applications where high temperatures are present, but high strength is still needed and high density is not an issue.[17] Tungsten wire filaments power the vast majority of household incandescent lamps, but are also widely used in industrial lighting as electrodes in arc lamps. Lamps become more efficient at converting electrical energy into light at higher temperatures, and therefore a high melting point is important for filament applications.[18]Gas tungsten arc welding (GTAW, also known as tungsten inert gas (TIG) welding) in The equipment uses a permanent, non-consumable electrode. Its high melting point and wear resistance against electric arcs make tungsten a suitable electrode material.[19][20]
Tungsten's high density and strength are also key characteristics for use in weapons. shells, for example, as an alternative to depleted uranium for tank guns.[21] Its high melting point makes tungsten a good material for applications such as rocket nozzles, such as the UGM-27 Polaris.[22] Some uses of tungsten are not related to its refractory properties, but simply to its density. For example, it is used in balancers for airplanes and helicopters or for headgear. golf clubs.[23][24] Similar dense materials such as the more expensive osmium may also be used in these applications.
The most common use of tungsten is as a tungsten carbide compound in drills, machining and cutting tools. The largest reserves of tungsten are in China, with deposits in Korea, Bolivia, Australia, and other countries.
It also serves as a lubricant, antioxidant, in injectors and bushings, as a protective coating and in many other ways. Tungsten can be found in printing inks, x-ray screens, photographic chemicals,[ doubtful – discuss
] in the processing of petroleum products and fire resistance of textiles.
Niobium alloys
Main articles: Niobium § Applications, and Niobium alloy
Apollo CSM with dark niobium titanium alloy rocket nozzle
Niobium is almost always found together with tantalum and was named after Niobe, the daughter of the mythical Greek king Tantalus after whom tantalum was named. Niobium has many uses, some of which it shares with other refractory metals. It is unique in that it can be processed by annealing to achieve a wide range of strengths and ductility, and is the least dense of the refractory metals. It can also be found in electrolytic capacitors and in most practical superconducting alloys. Niobium can be found in aircraft gas turbines, vacuum tubes and nuclear reactors.
The alloy used for liquid rocket thruster nozzles, such as in the main engine of the Apollo Lunar Modules, is C103, which is composed of 89% niobium, 10% hafnium and 1% titanium.[25] Another niobium alloy was used for the Apollo Service Module nozzle. Because niobium oxidizes at temperatures above 400 °C, these applications require a protective coating to prevent the alloy from becoming brittle.[25]
Tantalum and its alloys
Main articles: Tantalum and Tantalum § Applications
Tantalum is one of the most corrosion resistant substances available.
Due to this property of tantalum, many important applications have been found, especially in medical and surgical fields, as well as in harsh acidic environments. It is also used to make high quality electrolytic capacitors. Tantalum films occupy the second place in terms of capacity per volume of any substance after Airgel, [ citation needed
] and allow miniaturization of electronic components and circuitry. Many cell phones and computers contain tantalum capacitors.
Rhenium alloys
Main article: Rhenium
Rhenium is the most recently discovered refractory metal. It is found in low concentrations with many other metals, in ores of other refractory metals, platinum or copper ores. It is used as an alloy with other refractory metals, where it adds ductility and tensile strength. Rhenium alloys are used in electronic components, gyroscopes and nuclear reactors. Rhenium finds its most important use as a catalyst. It is used as a catalyst in reactions such as alkylation, dealkylation, hydrogenation and oxidation. However, its rarity makes it the most expensive of the refractory metals.[26]
The hardest metal
Titanium is considered the hardest and lightest metal on our planet. Due to its properties, it is actively used in aviation and shipbuilding - the material is excellent for the manufacture of aircraft and ship hulls. In addition, due to its strength and lightness, body armor is made from titanium. This metal is safe for the human body, therefore it is often used in medicine for the manufacture of instruments and even prostheses - artificial body parts.
Due to its outstanding properties, the word “titanium” is used to describe video cards and other electronics to emphasize their power.
When heated, titanium begins to absorb oxygen, chlorine, nitrogen and other gases. Thanks to this amazing property, the metal is used in various filters - passing various gases through titanium tubes heated to 600 degrees Celsius, you can clean them of impurities. In the same way, you can purify water from oxygen, which is especially useful in the food industry. It is believed that the oxygen contained in water degrades the quality of some products - at a minimum, it can shorten the shelf life of beer.
Advantages and disadvantages
Refractory metals and alloys attract the attention of researchers for their remarkable properties and promising practical usefulness.
The physical properties of refractory metals such as molybdenum, tantalum and tungsten, their strength and high temperature stability make them suitable materials for hot metal working applications and for vacuum furnace technology. These properties are used in many special applications: for example, tungsten lamp filaments operate at temperatures up to 3073 K, and molybdenum furnace windings can withstand temperatures up to 2273 K.
However, poor processability at low temperatures and extreme oxidation at high temperatures are disadvantages of most refractory metals. Interaction with the environment can significantly affect their creep resistance at high temperatures. The use of these metals requires a protective atmosphere or coating.
Refractory metal alloys of molybdenum, niobium, tantalum and tungsten have found application in space nuclear power systems. These systems have been designed to operate at temperatures ranging from 1350 K to approximately 1900 K. The environment must not interact with the material in question. Alkali metal liquid because heat transfer fluids are used, as well as ultra-high vacuum.
High temperature slug voltage alloys must be limited for their use. Creep strain should not exceed 1-2%. An additional challenge when studying the creep of refractory metals is the interaction with the environment, which can significantly affect creep.
Tungsten is the most refractory metal
Tungsten is a simple chemical substance, an element of the periodic table, a transition metal. It is written as the Latin letter W. It gets its name from the mineral wolframite, known to miners since the 16th century. The mineral Wolf Rahm itself (translated from German as “wolf foam”) got its name because it made it difficult to obtain tin from tin-containing rocks. During smelting, tungsten impurities formed compounds with tin and rose to the surface in the form of foam; in the language of the miners, “they devour tin like a wolf devouring a sheep.”
It is a rare element and ranks 57th in terms of prevalence on the planet. It is found only in minerals consisting of compounds of different metals. In the mining industry, the most significant are wolframite, scheelite, ferbelite, and hübnerite. In deposits, the concentration of tungsten rarely exceeds 2%.
Tungsten was discovered as a separate chemical element at the end of the 18th century. The famous Swedish chemist K. Scheele conducted experiments with the mineral tungsten (later renamed in his honor and called scheelite). Having treated it with nitric acid, the scientist found out that the sample was a salt of an unknown acid. His students continued working with the interesting mineral and, after two years of persistent research, isolated a metal unknown to science, which they called tungsten. This discovery did not cause much noise, because... the new metal was extremely refractory and there were simply no furnaces in the world capable of providing the required temperature for smelting. But in the twentieth century, tungsten made a real revolution in industry.
| Electric furnaces with a maximum heating temperature of +1300 °C | Electric furnaces with a maximum heating temperature of +1100 °C |
Properties
Light silver shiny metal reminiscent of platinum. Very dense, heavy, hard, but at the same time fragile. Melts at about +3400 °C, this is the highest among metals. (Theoretically, seaborgium transactinoid may be more refractory, but this is a short-lived radioactive element No. 106, obtained artificially as a result of nuclear fusion.) Under normal conditions, tungsten is difficult to machine, but when heated above +1600 ° C it can be forged, rolled, drawn into thin thread. Paramagnetic (can be magnetized in an external magnetic field), has high electrical resistance.
In chemical reactions it can exhibit valencies from 2 to 6, but all stable compounds are formed by W(VI). At temperatures around +20 °C it does not corrode in water and air. Reacts very weakly with hydrochloric, hydrofluoric and non-concentrated sulfuric acids. But it interacts easily with hydrogen peroxide, nitric acid, a mixture of nitric and hydrofluoric acids, and aqua regia. At high temperatures and in the presence of oxidizing agents, it reacts with alkalis. Forms oxides, tungstates (salts of tungstic acids), compounds with halogens, sulfur, carbon.
Does not participate in the metabolism of animals and humans.
Tungsten dust, like dust of any other metal, has an irritating effect on the respiratory system.
This is interesting
On Earth, there are several types of archaebacteria and bacteria that use enzymes with tungsten in their metabolic processes. Scientists believe that their development dates back to the early Archean era (about 4 billion years ago), when this metal played an important role in the creation and development of life on the planet.
Tungsten is a highly sought after metal in industry. We will tell you more about its use in the next article.
Recommendations
- "International Journal of Refractory Metals and Hard Materials". Elsevier. Retrieved 2010-02-07.
- Bauccio, Michael; American Metal Society (1993). "Refractory metals". ASM Metals Handbook
. ASM International. pp. 120–122. ISBN 978-0-87170-478-8. - Metals, behavior; Wilson, J. W. (1965-06-01). "General behavior of refractory metals". Behavior and properties of refractory metals
. pp. 1–28. ISBN 978-0-8047-0162-4. - Davis, Joseph R. (2001). Alloying: Understanding the Basics
. pp. 308–333. ISBN 978-0-87170-744-4. - ^ a b
Borisenko, V. A. (1963).
"Study of the temperature dependence of molybdenum hardness in the range of 20–2500 ° C." Soviet powder metallurgy and metal ceramics
.
1
(3): 182. doi:10.1007/BF00775076. - Fathi, Habashi (2001). "A Historical Introduction to Refractory Metals". Review of Mineral Processing and Extractive Metallurgy
.
22
(1): 25–53. Doi:10.1080/08827509808962488. - Schmid, Kalpakchyan (2006). "Slug". Manufacturing Technologies and Technologies
. Pearson Prentice Hall. pp. 86–93. ISBN 978-7-302-12535-8. - Weronski, Andrzej; Hejwowski, Tadeusz (1991). "Creeping materials". Thermal fatigue of metals
. CRC Press. pp. 81–93. ISBN 978-0-8247-7726-5. - Smallwood, Robert E. (1984). "Molybdenum alloy TZM". ASTM Technical Special Publication 849: Refractory Metals and Their Industrial Applications: A Symposium
. ASTM International. clause 9. ISBN 978-0-8031-0203-3. - Kozbagarova, G. A.; Musina, A. S.; Mikhaleva, V. A. (2003). "Corrosion resistance of molybdenum in mercury". Metal protection
.
39
(4): 374–376. Doi:10.1023/A: 1024903616630. - Gupta, K. K. (1992). "Electrical and electronic industry". Mining metallurgy of molybdenum
. CRC Press. pp. 48–49. ISBN 978-0-8493-4758-0. - Magyar, Michael J. "Commodity Review 2009: Molybdenum" (PDF). US Geological Survey. Retrieved 2010-04-01.
- Erwin, D. R.; Bourell, D. L.; Persad, C.; Rabenberg, L. (1988). "Structure and properties of high-energy, high-strength consolidated molybdenum alloy TZM." Materials Science and Engineering:
A.
102
: 25. Doi:10.1016/0025-5416(88)90529-0. - Oleg D., Neykov (2009). "Properties of molybdenum powder and molybdenum alloys." Handbook of Non-Ferrous Metal Powders: Technologies and Applications
. Elsevier. pp. 464–466. ISBN 978-1-85617-422-0. - Davis, Joseph R. (1997). "Refractory metals and alloys." ASM Specialty Handbook: Heat Resistant Materials
. pp. 361–382. ISBN 978-0-87170-596-9. - Lassner, Eric; Schubert, Wolf-Dieter (1999). Tungsten: properties, chemistry, element technology, alloys and chemical compounds
. Springer. pp. 255–282. ISBN 978-0-306-45053-2. - National Research Council (USA), Tungsten Panel, Committee on Technical Aspects of Critical and Strategic Materials (1973). Tungsten Trends: Report
. National Research Council, National Academy of Sciences - National Academy of Engineering. pp. 1–3.CS1 maint: several names: list of authors (link) - Lassner, Eric; Schubert, Wolf-Dieter (1999). Tungsten: properties, chemistry, element technology, alloys and chemical compounds
. Springer. ISBN 978-0-306-45053-2. - Harris, Michael K. (2002). "Welding Health and Safety." Welding Health and Safety: A Practical Guide for OEHS Professionals
. AIHA. paragraph 28. ISBN 978-1-931504-28-7. - Galvery, William L.; Marlow, Frank M. (2001). Welding Basics: Questions and Answers
. Industrial Press Inc. p.185. ISBN 978-0-8311-3151-7. - Lanz, W.; Odermatt, W.; Weihrauch3, G. (7–11 May 2001). KINETIC ENERGY PROJECTS: HISTORY OF DEVELOPMENT, STATE OF TECHNOLOGY, TRENDS
(PDF). 19th International Symposium on Ballistics. Interlaken, Switzerland. - Ramakrishnan, P. (01/01/2007). "Powder metallurgy for the aerospace industry." Powder Metallurgy: Processing for the Automotive, Electrical/Electronic and Mechanical Engineering Industries
. New Age International. paragraph 38. ISBN 81-224-2030-3. - Arora, Arran (2004). "Heavy tungsten alloy for defense purposes." Materials Technology
.
19
(4): 210–216. - Moxson, V.S.; (Sam) Froes, F. H. (2001). “Manufacture of sports equipment components using powder metallurgy.” JOM
.
53
(4): 39. Bibcode:2001JOM…. 53d..39M. Doi:10.1007/s11837-001-0147-z. - ^ a b
Hebda, John (2001-05-02).
"Niobium Alloys and High Temperature Applications" (PDF). Niobium Science and Technology: Proceedings of the Niobium 2001 International Symposium (Orlando, Florida, USA)
. Companhia Brasileira de Metalurgia e Mineração. Archived from the original (pdf) on December 17, 2008. - Wilson, J. W. (1965). "Rhenium". Behavior and properties of refractory metals
. Stanford University Press. ISBN 978-0-8047-0162-4.
Records for inorganic substances
The strongest stable oxidizing agent
, is a complex of krypton difluoride and antimony pentafluoride. Due to its strong oxidizing effect (oxidizes all elements to higher oxidation states, including oxygen and nitrogen in the air), it is very difficult for it to measure the electrode potential. The only solvent that reacts with it slowly enough is anhydrous hydrogen fluoride.
The densest substance
, is osmium. Its density is 22.5 g/cm3.
The lightest metal
- this is lithium. Its density is 0.543 g/cm3.
The most expensive metal
- this is Californian. Its current cost is $6,500,000 per gram.