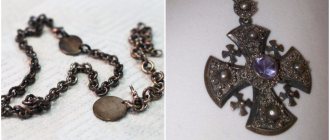The fear of being deceived haunts many people, and this fear is not unfounded. According to statistics, over the past 10 years the production of precious metals has practically not increased, but at the same time the number of jewelry stores is growing at an incredible pace. Alas, even when buying jewelry in a jewelry store, there is a chance of running into a low-quality fake. Are you overcome by doubts? Verifying the authenticity of precious metals is available not only to specialists, but to each of us. There are many ways to do this, which can be done at home.
additional information
St.12Х18Н9 (AISI 304) gives a short spark, colored light yellow with a few red dots appearing from time to time. At the point of contact with the abrasive and at the ends of the branching, the spark beam has a red-yellow color.
St.X12F1 - yellow, short spark, multiple “stars”, the ends are elongated in a line. The area where the abrasive touches is red-yellow. Individual red dots throughout the entire beam.
How to identify non-ferrous metal?
Today, many owners of private houses are trying to make the most of their own capabilities. We are talking about organizing a workshop. The presence of even a primitive lathe will allow you to produce a wide variety of parts for a wide variety of purposes. In any case, repairing garden equipment or even your own car will become much easier.
Meanwhile, savings can be achieved by purchasing metal raw materials on the most favorable terms. Today on the Internet you can find the most attractive prices for aluminum rods. Many people purchase metal for further use at scrap metal sites. Here the question arises - how to distinguish ferrous metal from non-ferrous metal?
The actual difference between ferrous and non-ferrous metals
Surveys show that the average person cannot answer this question. The essence of the answer lies in the composition of the metals.
Ferrous metals contain an admixture of iron. Therefore, ferrous metal can be determined using an ordinary magnet. If it sticks to a metal part or workpiece, we can say with confidence that it is made of ferrous metal.
However, there are exceptions to any rule. Nickel also attracts a magnet, although it is considered exclusively non-ferrous metals. Additionally, the magnet rule does not apply to some metal alloys.
These include stainless steel.
Kinds
The large number of non-ferrous metals and various characteristics required their classification into separate types.
industrial systematization is in use , reflecting the historically established components of the metallurgical industry and the science of the same name.
The name itself does not fully reflect the essence of non-ferrous metal.
Only gold and copper are colored, while the rest are the usual grey-black shades.
Science usually distinguishes the following types of non-ferrous metals and alloys:
- lungs;
- heavy;
- noble;
- refractory;
- scattered;
- rare earth;
- radioactive.
industry in Russia today is on the rise and includes:
- metal mining;
- ore beneficiation;
- metal smelting
Basic non-ferrous metals
The main non-ferrous metals include:
- copper;
- aluminum.
Aluminum is an excellent electrical conductor. It is flexible, which is both its advantage and disadvantage.
To give it strength add :
- manganese;
- copper;
- magnesium, etc.
Such alloys are used for the production of :
- airplanes;
- sea and river ships;
- space shuttles;
- in construction;
- in the food industry.
Aluminum and its alloys are the cheapest type of non-ferrous metal scrap.
You can find it in a variety of household items, including:
- siding;
- gutters;
- roofing
Copper is a commonly found non-ferrous metal.
It also has good characteristics:
- plastic;
- good electrical conductor;
- good heat conductor.
It is in great demand in alloys and is used in various economic sectors.
Its alloy with zinc and tin is known - brass.
It can be found in:
- cars;
- hours;
- expensive jewelry.
find copper for scrap metal in:
- power cables;
- water pipes;
- household products.
Copper is highly valued at recycling centers.
Rare
Rare earth metals are used to improve the qualities of other metals and became widely used with the development of industrial production in the 20th century.
These are the following metals:
- scandium;
- yttrium;
- lanthanides.
The name itself suggests that there is very little of these non-ferrous metals in the earth's crust. Also, previously, refractory oxides that form rare non-ferrous metals were called “earths” . They are extracted from oxides.
Today, rare earth metals can be found in all digital devices:
- smartphones;
- players;
- computers;
- in hybrid engines;
- in other electronics.
Alloys made from them have high characteristics , for example:
- anti-corrosion;
- strength;
- heat resistant.
Heavy
Let's consider heavy non-ferrous metals, collecting them in several lists.
The heaviest non-ferrous metals on Earth:
- osmium;
- iridium.
Rarely found in soil , it is generally the most expensive non-ferrous metal.
Also included in this group are:
- copper;
- lead;
- zinc;
- tin;
- nickel.
All of them have a high density and, accordingly, a lot of weight, which is why they get the name – heavy.
Lead is widely known and used in many industries , contained in:
- rock
- soil.
It is made from:
- batteries;
- explosives;
Lead is also used to create protective aprons from radiation .
Has the following characteristics:
- low thermal conductivity;
- plastic;
- toxicity.
Therefore, lead must be used carefully, following all safety regulations.
Tin used to be called an alloy of lead and silver.
Today, tin is used in the metallurgical industry and the production of various alloys, which include:
- bearings;
- packaging foil;
- bronze;
- food tin;
- wires
Nickel is a heavy non-ferrous metal with high heat-resistant and anti-corrosion characteristics. Nickel is used in alloys. In stainless steel it is the main component.
Made from nickel :
- coins;
- armor;
- chemical equipment;
- wire;
- foil;
- a thread;
- powder;
- alkaline batteries.
in demand in:
- shipbuilding;
- electrical engineering.
Lungs
The definition of “light non-ferrous metals” includes metals with low density.
List of the most popular light non-ferrous metals:
- aluminum;
- tin;
- magnesium;
- titanium;
- beryllium;
- lithium.
The lightest non-ferrous metal is lithium. It is widely used in various alloys.
is used in:
- chemical industry;
- metallurgical industry;
- military-industrial complex;
- thermonuclear energy.
Lithium is also used in the manufacture of:
- optics;
- alkaline batteries;
- ceramic products.
The ductility of magnesium is not as good as that of copper and aluminum, which affects the welding properties of this metal. But it can be easily cut with a special tool. At the same time, the mechanical properties leave much to be desired. This it to be widely introduced into industrial production .
Non-ferrous metals that are not magnetic
These can safely be attributed to:
- aluminum;
- copper;
- brass;
- bronze
However, there is an easier way to identify ferrous metal. If you purchase metal at a dealer (reception point), it is very likely that it is located in the open air.
We are talking about the fact that the metal is affected by precipitation. This means that it is in no way protected from corrosion. Consequently, ferrous metals immediately begin to become covered with rust.
Non-ferrous metals are a priori not subject to corrosion (under the influence of the environment). They are protected by an invisible oxide film that appears on the surface.
The video explains how to recognize metal (non-ferrous or ferrous):
Source: euroelectrica.ru
How to tell the difference?
Most often you can distinguish by:
- mind;
- weight;
- degree of hardness
without the use of any tools or equipment.
But there are situations when, for accuracy, it is necessary to use :
- reagents,
- tools,
- devices.
Before assessing the scrap that you are going to take to the collection point, you need to clean it of dirt, otherwise you won’t be able to accurately determine it by eye.
By color
Both metals, although to varying degrees, can develop a patina .
Therefore, do not forget to clean the scrap well.
If an object has been in the open air or in water for a long time, the patina layer is difficult to remove.
Sometimes it will be justified to purchase a special cleaning product .
It is advisable to inspect the scrap under a powerful white light.
This implies that one can view either under the sun on a fine day or under a bright fluorescent lamp . Incandescent lamp is not suitable.
Pure copper will have a reddish-brown tint, sometimes with a pink tint. Keep in mind that brass can be red or orange. This type is commonly used for decorations and water pipes.
If the material has an orange, yellow or golden tint, you can be almost sure that it is brass.
If you are engaged in the collection and delivery of scrap metal, then it will be useful for you to know the prices for scrap ferrous metals. If you don’t know where to find ferrous metals, then read this article. Not sure which metal detector model to choose? Check out our review of popular models.
It can also be light golden , pale yellow , and even off-white , but it is very rare for metal detectors, since such an alloy is difficult to process, and it is used mainly in jewelry.
The best recommendation is to carry an item that you are absolutely sure is made of pure copper . You will be able to compare it with the crowbar you found. Most often this method works well.
By sound
Another method that does not require special skills or equipment. You can learn to distinguish metals by sound after a short training. Hit the object with something metal. If it is made of copper, the sound will be muffled and low . This happens because the metal is soft.
Visual inspection and testing for sound and hardness are usually sufficient for field determination.
On the contrary, brass will produce a ringing and high-pitched sound when struck. The second most important inspection method for those who deal with scrap metal is after visual assessment in the light. But this method is justified only with large and voluminous objects - you need something to make a sound.
By hardness
Copper, as mentioned above, is a soft metal. Brass was specifically created to increase the hardness of copper while maintaining some of its other characteristics. Therefore, when damage is caused to scrap, copper will be the material that is more easily deformed . Brass, on the other hand, can withstand impacts .
By labeling
If an item has markings on it, identifying the metal or alloy can be simple and accurate.
As a rule, brass is marked with a mark that begins with the symbol “L” .
Accordingly, copper markings begin with “M” . True, copper quite often does not have any markings.
Here are some transcripts that may be useful:
- Copper markings begin with one letter “M” , followed by numbers. letter “L” on brass products; it can be followed by more letters, and only then by numbers.
- In the United States and Canada, the UNS , according to which brass is marked C2, C3, C4 .
- In the European Union, both metals are marked with the letter C , it all depends on the subsequent letters. For copper they will be B , C, D , and for brass alloy - L, M, N, P and R.
- Not so long ago, labeling consisting of icons of chemical elements was common. For example, Cu Zn (cuprum - zinc) will mean brass.
By weight
Brass is lighter than copper due to the addition of zinc. But in order to determine from a shapeless piece whether it is metal or an alloy, experience is required.
By chips
This test will require a metal drill or access to a machine to get the chips.
With brass it will be, as experts say, needle-shaped , since the material is hard.
It's kind of loose.
With copper, the shavings will be more plastic, so often they don’t even break and the result is ornate , in one continuous spiral.
However, there are grades of brass whose shavings are similar to copper. According to reviews from practitioners, the LS63 grade is a very plastic and tough alloy; after its processing, spiral chips remain.
Acid analysis
If you come across brass grade L-96 , which means the presence of 96% copper in the alloy, it is difficult to distinguish it from the metal without analysis. You can use hydrochloric acid for this. If you drop it on pure copper, it will simply cleanse it of patina and will not react with the metal itself.
If you apply hydrochloric acid to brass, zinc will react and a white oxide will appear on the surface - zinc chloride .
Analyzer
Our portal has detailed material about analyzers of metals and alloys. With the help of such a device you can accurately determine what is in front of you.
Such analyzers have a liquid crystal screen, on which, after interaction between the device and the metal object, a complete list of all constituent elements is displayed.
If it is 99% copper and tenths of a percent of some random impurities, it is copper. If there are other metals in significant quantities - brass. But the method is expensive .
By product type
Some products are made only from copper or only from brass.
This may provide additional guidance.
Tools are made exclusively from brass, which is harder.
Some parts of wind musical instruments are made from copper.
In principle, you need to start from the purpose of the item - if it should be:
- reliable,
- hard,
- stiff,
then, most likely, brass .
If, on the contrary, you need ductility , high electrical or thermal conductivity, then this is copper .
By heating
Another method in which you need to use a gas burner.
The indicator here will be zinc oxide, which forms as a pale white ash-colored coating only on brass if it is heated to temperatures above 600 degrees .
Methods for determining metal
Probably everyone had to hold in their hands a piece of jewelry or another object, obviously metal. But how can you determine what metal is used in production? It could be a precious material or a counterfeit, or even a trinket with no claims to value. Expertise from specialists will give you the exact answer, but it is not free. But there are methods for approximately determining the type of metal at home. They were used a long time ago, but they have not lost their relevance in our time.
Authentication of platinum jewelry: your own expert
Platinum is a precious metal used in jewelry making. The silver-white metal gained its fame back in the 18th century, but only in our time has it become widely used by jewelers. As a rule, only small jewelry such as rings, earrings and chains are made from platinum. This is due to the high cost of the material.
With the spread of the Internet, purchasing jewelry has become much easier. However, you will not have the opportunity to examine the product before purchasing, which means you may encounter a fake. Many people prefer not to take risks by purchasing jewelry in specialized stores, but even in this case you are not immune from counterfeiting. So how can you independently verify the authenticity of jewelry made from platinum?
Determination of product weight and density
Platinum is a heavy metal, the weight of which is comparable only to iridium, osmium, rhenium and uranium. All other elements are easier. In addition, in the production of jewelry, the specific gravity of platinum ranges from 85% to 95% of the total weight of the product. That is, jewelry is almost 100% made of this noble metal. Products made of gold and silver, for example, contain much less precious metal.
The use of iridium, osmium and rhenium to make jewelry heavier is inappropriate, since these elements have the same cost as platinum, and they are rarely found in nature. Pick up a platinum ring and a similar sized ring made of another metal. A product made of platinum will be heavier than a similar piece of jewelry made from a different alloy.
May 28, 2016 at 8:30 PDT
Real silver darkens over time. But this takes years, and besides, its shine can be easily restored. To do this, use a special cream or ammonia. The shine of low-quality products disappears forever.
Silver test
One of the most accurate methods for determining the authenticity of silver at home is a silver test, which can be purchased in specialized stores or online. By following simple instructions, with a high degree of probability you can determine not only the authenticity of the product, but also the approximate sample.
There are electronic devices that can distinguish silver, gold, white gold, and platinum. However, their cost is so high that purchasing them for the purpose of one-time determination of precious metals at home is not economically justified.
Magnet check
Bringing a magnet close to the item being tested is a good way to perform initial testing. By the reaction of the magnet you can determine which group the metal belongs to:
- Ferromagnets. The magnet is clearly attracted to the object, which means that the product may contain iron, steel or nickel.
- Paramagnetic materials. The interaction with the magnet is very weak. This group includes aluminum and chrome. Precious metals that are paramagnetic are platinum, palladium and silver.
- Diamagnets. In general, they do not react to magnets. Copper and zinc have these properties. Precious metals - gold.
Of course, such a check will not allow us to accurately determine the material from which the item is made. After all, a non-magnetic metal may not be in its pure form, but in the form of an alloy with a ferromagnet. But it can confirm or refute the assumption. For example, if it is checked whether it is gold or not, but the item is clearly magnetic, then it can be argued that it is a fake.
When checking jewelry, you should take into account that, in addition to precious metals, they may contain clasps, built-in springs, made of another material. You need to check the metal itself.
Heat check
You can also determine the group of a metal by how it conducts heat. It is known that the thermal conductivity of silver is very high. It is almost five times higher than that of iron or platinum. Slightly worse for gold, copper and aluminum. Platinum transfers heat even weaker than iron.
If you immerse the metal in hot water for 15–20 seconds, then based on its temperature, determined by touch, you can draw some conclusions.
- Gold and silver objects will become as hot as the water in which they were dipped.
- During this time, platinum and items containing iron will become warm, but not hot.
In this way it is easy to distinguish platinum from silver. But it’s not possible to compare silver or aluminum alloy.
Similarities and differences
Brass alloy consists mostly of copper, so it is natural that they are similar not only visually, but also in some properties. The more copper in the alloy, the more similar their colors will be. This is where the exact coincidences end.
Visually, less than 80% copper are easily distinguished . They are slightly similar to gold, as they have a pronounced yellow tint. The more zinc, the lighter the shade.
Because of this, brass is even used to counterfeit or imitate gold . Copper has a main shade of reddish, which often has a pink tint.
With a strong decrease in temperature, brass does not lose its relatively limited ductility and does not become brittle . Conducts electricity and heat worse.
They differ in such a way as hardness .
Copper is softer and more ductile , while brass, on the contrary, is hard and it is difficult to give it any shape without annealing.
The shavings are also different: for brass they are needle- shaped , for copper they are twisted into a spiral .
Let's look at the properties that brass and copper have and whether they have any differences:
| Copper | Brass |
| Plastic, soft | Solid |
| Reddish-brown-pink tint | Golden tone |
| Lower sound on impact | Alt |
| Heavy | Easier |
| The shavings are twisted into a spiral | Needle shavings |
Iodine test
You can check the authenticity of the metal using an iodine solution purchased at a pharmacy. A drop of iodine is applied to the surface and left for several seconds. Iodine will not harm noble metals - gold, platinum, silver. If the color of a drop of iodine does not change, and after removing it with a napkin, no traces or stains remain, this indicates the authenticity of the metal. If darkening is visible at the place of the drop, then this is a low-quality alloy or an outright fake.
Vinegar test
Household vinegar solution also does not affect precious metals. And it is dangerous for counterfeits. But, unlike the iodine test, acetic acid takes time. To wait for the result, you need to immerse the metal being tested in a container with vinegar for 15–30 minutes. The absence of traces of interaction between metal and vinegar is a sign of nobility.
If, in addition to metal, the product contains precious or semi-precious stones, then it is better not to check them this way; vinegar can ruin them. This is especially true for pearls.
Dental check
From novels and films we know that they used to test the authenticity of gold coins by biting them. What exactly can be installed in this “old-fashioned” way? Gold is a soft metal. Therefore, even with a weak bite, a dent from the teeth remains on it. Fake alloys do not have this property; you cannot take them with your teeth.
Such a test gives good results for high-quality products. The higher the pure gold content, the softer it is. Gold of 900 purity and higher is so soft that they try not to expose valuable items made from it to contact with other objects.
Application of chemicals
Testing with active chemical reagents should be left as a last resort. If handled improperly, they will damage even genuine precious metal. And they can be dangerous for the health of the inspector.
Pure gold does not react to ammonia. But practically no products intended for use are made from gold 900 and 999, only for collections. And on a precious metal of lesser purity, ammonia can leave an irremovable mark. Its solution in combination with other substances is used to clean gold items. Therefore, it is not worth identifying gold and silver items using ammonia.
What is better for health, gold or silver?
In terms of health benefits, silver products are better than gold ones. In what cases should men and women wear them? Silver jewelry looks less provocative than gold. Any massive earrings or bracelets will not seem vulgar if they are made from this material.
Interesting materials:
How much does a Rolls Royce figurine cost? How much does a taxi cost to Domodedovo airport? How much does Theraflu powder cost? How much does it cost to install sockets? How much does it cost to bury an urn in a columbarium? How much does it cost to replace a license through government services? How much does a WWII subscription token cost? How much does a golden chinchilla cost? How many pages are in the book Tom Sawyer? How many pages are there in the Red Book?
Density check
One of the reliable ways to determine the type of metal or alloy is to determine its density. For pure gold it is two times higher than for copper and almost three times higher than for iron. Platinum is even heavier than gold. Even an alloy of 585 gold is noticeably heavier than base metals.
Of course, to determine the exact density of a small product you will need pharmaceutical scales, volume calculations (Archimedes' law to help) and tabular data on the density of base metals. But to solve the question of what the alloy is mainly made of, gold or another metal, rough estimates are sufficient. If you have at hand an object made of obviously genuine metal of approximately equal volume, then you may not even need a scale. A weight difference of two to three times is not so difficult to catch.
Separately, each of the considered methods will not give an exact answer to the question of what metal the product is made of. But if several different tests show the same results, you can be confident in the correct determination. If not, then you will have to turn to professionals.
Source: dedantikvar.com
How to determine the type of metal at home?
Recycling and reusing items such as plastic bottles, worn-out clothing and newspapers is very important and forms part of everyday life, helping to increase your contribution to the green movement. One segment of the recycling industry that may not be as popular, but is no less important, is scrap metal recycling. The export of scrap metal is widespread in the Russian Federation and, by processing metal, we reduce the volume of ore production throughout the world.
Some of these metals include copper, steel, aluminum, iron and wire, but are often simply thrown into the trash due to a lack of knowledge and sources of metal recycling. We are here to help educate the public about the possibilities of collecting certain metals and taking them to the right place.
Scrap metal recycling makes money
What many people don't know is that most scrap metal can be recycled for a fee at local scrap metal collection points across the country, thus joining the green movement.
Scrap metal collection centers work with customers from the trade industry who deal with metal every day. Many of them are construction companies that have tons of metal beams from structures, electrical companies that have wires and electrical equipment, or plumbers that have copper piping and brass fixtures. While scrap yards receive a huge amount of such metals from the trading industry, ordinary people are also welcome and encouraged to bring their scrap metal, get paid and have the metal recycled at the right place.
Magnet evaluates the value of metal
Identifying and separating ferrous and non-ferrous metals is the first important step before you recycle the metal. The simplest and most accessible way to determine metal is to test it with a magnet.
Tip: If you don't have a portable magnet on hand, any magnet will do - even one from your refrigerator.
If a magnet is attracted to your metal: You are holding a ferrous (ferrous) metal, something as simple as steel or iron. Ferrous metals don't cost much at scrap yards, but they will be accepted to make sure the metal is recycled properly.
If the magnet does not stick to the metal: You have iron-free metal. Many common metals such as copper, aluminum, brass, stainless steel and bronze are classified as non-ferrous metals. These metals are very valuable for recycling and they pay more for them at scrap metal collection points.
So you've got your metal sorted, now call the metal collection center and ask what metals they accept to get an idea of the procedure before you go there yourself. Homeowners are often apprehensive about going to scrap yards, but making sure your scrap metal is carefully sorted will take a step in the right direction. Some places recommend that you bring the scrap metal to them and put it on a scale, while others will remove it all themselves.
This will help you identify the metal:
The most difficult part of metal recycling is determining its grade and value. Knowing these basic metals will make the task much easier:
In good condition it has a reddish color, and if it is quite worn out, the color may be dark brown with green rust spots. Find out how to recycle copper and other scrap metals using the contact section. Copper is a common material in home construction. It can be found throughout the home as plumbing pipes, roof drainpipes, as well as internal parts of air conditioners and general electrical wiring. Copper can also be found in electrical wires: under black or colored braiding there is brightly colored copper wire. Copper is one of the most valuable materials to recycle, so collecting it and storing it separately from other metals can net you some monetary rewards.
Aluminum is usually painted white, but it is a whitish silver color and is easy to bend if it is thin. Aluminum cans are not the only source of this metal. While cans are collected and brought to scrap yards by the stack, aluminum can also be found around the home. Often used for gutters, siding, window frames, doors and more, aluminum can be found more often than you might think. Although aluminum is not very expensive at recycling centers, most of it can be recycled and reused within a few months. Recycling aluminum saves 80% of the energy spent on its production.
General concepts about brands
We will consider markings that were developed back in the USSR and are now actively used in Russia and in all neighboring countries. It is universal in that it includes all classes, of which there are a lot. Basic moments:
- The number is assigned to the entire batch, and a stamp is affixed (paint, by engraving) to each individual product. It consists of numbers and letters, no symbols.
- Sometimes the abbreviation “St” is indicated at the very beginning, that is, “steel,” but this is not at all necessary.
- Typically, the initial numbers indicate hundredths of carbon, while the letter designating this substance is not placed, since carbon content is one of the fundamental characteristics of alloys. For example, if 20 is indicated, then the content is 0.2%.
Now in more detail with an example:
We have letters (Russian or Latin, as in the sample), they indicate the alloying element that is in the composition. If you need a method for determining the grade of metal without reference books, then you will need to fill in the most common abbreviations:
- A – nitrogen.
- N – nickel.
- X – chrome.
- T – titanium.
- K – cobalt.
- B – tungsten.
- C – zirconium.
- C – silicon.
- D – copper.
- B – niobium.
- G – manganese.
- Yu – aluminum.
A more complete list can be found in regulatory documents. By the way, it is interesting that GOST standards for the production of steel alloys, adopted back in the Soviet Union, are still in force today, as are the marking rules. In total, the nomenclature includes more than 1,500 individual values - this is the number of varieties of metals in this category that are manufactured all over the world. It is not surprising that in such a variety it is very difficult to determine by eye what kind of material is in your hands.
We figured out the letters, now the numbers. Everything is simple with them - the first one belongs to carbon, and then we read from left to right: a letter, and behind it a digital indication - what proportion (as a percentage) of the substance is in the composition.
But, in addition to the designation of chemical elements, you can also find other, sometimes incomprehensible, letters. They may indicate the presence of special properties, as well as membership in any category. Let's look at how to check the steel grade using these designations below.
Material quality
In addition to additives specially introduced in precise proportions that improve the quality of the alloy, there are harmful impurities - they are in the solution during smelting without the intention of the metallurgists. These are usually non-metals that have a negative effect. For example, phosphorus makes the metal very brittle when the temperature drops, and sulfur leads to the formation of cracks when heated. Therefore, they try to get rid of these and other elements (oxygen, excess nitrogen), and the less of them in the sample, the higher its quality. May be called:
- ordinary, then “St” is put at the very beginning, this means that the impurities are in the amount of 0.06-0.07%;
- qualitative - no special marks are placed, substance content - up to 0.035%;
- high quality - at the end of the marking there will be an “A” (not to be confused with nitrogen), this means that no more than 0.025% of harmful elements;
- especially high-quality - the name ends with the letter “Ш”, and the percentage does not exceed 0.02%.
In addition, when working with an ordinary quality class, it is also necessary to take into account the categories - from 0 to 6. This results in “St” with a digital index. The lower the number, the better the composition, in terms of impurities.
Another important concept is the degree of deoxidation. This is an indicator that reflects the behavior of the metal in the molten state. It depends on how oxygen is removed from solution. According to this classification, alloys can be:
- Calm (SP in the marking), they harden without gas evolution. They contain manganese, silicon or aluminum.
- Semiquiescent (SS), which are deoxidized in two stages due to the retained carbon.
- Boiling (KP). During their heating, carbon dioxide is actively produced, which rises to the surface in bubbles and thus solidifies.
And if you are dealing with alloy steel, you may come across specialized designations; for example, here are a few abbreviations:
- Ш – ball bearing purpose.
- R – high-speed cutting for making tools.
- A – automatic specialized.
- E - electrical, it is purified from virtually any impurities, more than 99% is solid iron.
Ways to determine the type of metal yourself
How to determine a metal and its origin? The question is mainly of interest to jewelry owners who are afraid of purchasing a fake for an impressive amount. You can deal with this problem yourself or contact an expert. The jeweler will conduct an examination, issue an opinion and charge a fee for the work. Authenticity testing can cost from 10 to 20% of the cost of jewelry. If you can’t contact a specialist, then you should try to solve the problem on your own.
Comparison of platinum, silver and white gold
All that glitters is not gold
Only a jeweler who has all the necessary analytical equipment can check a gold item and issue a reliable conclusion as to whether it belongs to a noble metal. Professional verification is carried out by the Assay Chamber. Examination of gold jewelry is not a cheap pleasure; the price of the service ranges from 10 to 20% of the estimated value of the item. Gold is being counterfeited more and more often, and no one wants to waste money. By the way, the need to check gold for authenticity may arise not only in relation to jewelry, but also, for example, when purchasing bars or nuggets.
Silver earrings with cubic zirconia, SL; (price on the link)
The most difficult type of counterfeit to independently identify gold is a piece of jewelry on which a thin layer of precious metal is applied. It is extremely difficult to determine the authenticity of such work at home without causing damage to the product.
The most common methods of counterfeiting gold items:
- surface gilding;
- replacement with copper;
- alloys of aluminum and other metals;
- an alloy of titanium and gold.
Fake jewelry made from alloys similar in color to gold leave spots on the skin with a green tint, especially when the ring is worn for a long time. The substitution of less valuable metal alloys for gold by others or similar deposition can be determined using well-known methods.
The first stage is checking gold by comparison. Surely you have a piece of jewelry whose authenticity you have no doubt about. A line should be drawn on a hard object with these two decorations. Gold items will leave the same mark, but if there are differences, this is a direct reason to doubt the quality.
Using a magnifying glass, look closely at the mark, which should reflect the gold standard. It should be clear and without damage.
The cost of a gram of gold changes daily, however, you should rely on it when purchasing jewelry, even if it is not purchased in a store.
Gold ring with diamonds and citrines, SL; gold earrings with diamonds and citrines, SL; (price on the link)
There is also an opinion that sound helps to identify a copy. Gold items emit a crystal ringing sound when they hit a hard surface. A dull or any other sound is a cause for concern.
Iodine test
Iodine can change the color of most components that are used to falsify precious metal, but this test is completely harmless for jewelry with a purity above 500 (i.e., which contains more than 50 mass percent gold).
A drop of pharmaceutical alcohol solution of iodine should be applied to the product that is in doubt, and after 10-15 seconds, remove its remains with a napkin. If a trace of iodine remains, then this is not a gold product. Unaltered metal color may indicate authenticity.
Magnet check
Precious metals are not affected by magnets. Steel products coated with a thin layer of gold will instantly be attracted to the surface of a magnet; real gold jewelry will not react to a magnet.
Many manufacturers use lock designs for chains and bracelets that include a steel spring - in this case, the magnet will only attract the lock.
Indifference to a magnet is a necessary condition, but not a sufficient one. For example, most alloys of copper and tin are non-magnetic. However, such products are much lighter: the difference in weight can be felt even without an analytical balance.
Vinegar test
A cheap fake will turn black when exposed to acetic acid. If you put gold with a purity above 500 into it, then nothing will happen to it. This is another surefire method of recognizing authenticity. 3-5 minutes is enough to conduct the experiment.
By the tooth
You've probably seen in films how the main characters tried a gold coin "by tooth." This method is only suitable for high-grade gold (whiter than 900), which is relatively soft. On such gold, teeth marks will definitely remain, since its hardness is much lower than that of other metals.
Determining the authenticity of gold using analytical instruments
Instant identification of metal components at home is possible using a special device called a metal analyzer. The result is displayed on the screen within 2-3 seconds. To obtain it, you need to point the device at the object under study. The analyzer is widely used by precious metal miners.
Gold earrings with diamonds and sapphires, SL; (price on the link)
In order not to doubt the authenticity of gold jewelry, you should choose the right place to purchase it. Jewelry stores and pawn shops are selling points for real precious metals. Buying second hand is always a risk.
How to check?
Owners of platinum jewelry should remember:
- Platinum is an expensive and heavy metal, and small jewelry is often made from it.
- Platinum can be replaced with silver, but such a substitution can be recognized by color.
- The hardest thing to distinguish from the original is jewelry that has a layer of platinum applied to it.
- The mark on the surface of the product should not raise doubts.
- Platinum is not afraid of high temperatures and reagents.
The cost of platinum is constantly increasing; there is not much of this metal in the world. Therefore, if in a store a buyer is offered to purchase an impressively sized product made of platinum, and its cost is quite low, he should refuse the purchase. Platinum is not sold cheaply, and jewelry made from it is small; the metal is too heavy.
Platinum Bank Bar
Silver and platinum are similar in appearance, so the expensive metal is often replaced with silver. Such a fake will give itself away with a black tint and plasticity. Silver is not resistant to damage; a mark will remain on its surface, but it will not be possible to spoil a platinum product in this way.
If a layer of platinum is applied to the surface of the product, then a fake can be recognized by its weight. When this is not possible, it will not be possible to determine its quality without causing damage to the jewelry.
Before purchasing, you need to carefully examine the mark; you can use a magnifying glass for this. If all the numbers are clearly visible, most likely the jewelry is really made of platinum.
Due to its chemical properties, platinum is not afraid of high temperatures and acids. When immersed in acid, ammonia and even when exposed to iodine, the product will not change. If you try to heat a ring or earring with a lighter, the temperature of the jewelry will not change immediately. Platinum is a poor conductor of heat, unlike silver.
General characteristics of metals
To share, click
In nature, metals exist in both free and bound forms. Low-active metals exist in free form: platinum, gold, silver. But mostly metals are found in the form of various compounds. Many metals are capable of reacting with each other. The products of the interaction of metals with each other are called alloys.
Metal atoms have a small number of valence electrons. They are weakly bound to the nucleus and can easily break away from it. As a result, positively charged ions appear at the nodes of the crystal lattice, and electrons move freely between them - the so-called “electron gas” is formed. The type of connection between positive ions, carried out due to the attraction of electrons moving freely throughout the crystal, is called metallic.
Metals have many specific properties. All of them, with the exception of mercury, under normal conditions are solid substances with a characteristic luster and conduct electricity and heat well. Most metals can be forged, drawn, and rolled. The electrical and thermal conductivity of a metal is explained by the presence of free electrons in it.
Metals are divided into light and heavy, differ from each other in hardness: the softest are alkaline, they can be cut with a knife; the hardest is chrome. Metals are divided into fusible and refractory. In metallurgy, metals are divided into ferrous (iron, manganese, chromium) and non-ferrous (all other metals).
Metals always act as reducing agents. The ability to donate electrons manifests itself in metals to varying degrees. The easier a metal gives up electrons, the more active it is and the more vigorously it interacts with nonmetals and ions of other metals. Based on their activity, all metals are arranged in a certain sequence, forming an electrochemical series of voltages.
Almost all metals have relatively large radii and a small number of electrons (from 1 to 3) at the outer energy level. Metals are characterized by low electronegativity values and reducing properties.
The most typical metals are located at the beginning of the periods (starting from the second), then from left to right the metallic properties weaken. In the group from top to bottom, the metallic properties increase as the radius of the atoms increases (due to an increase in the number of energy levels). This leads to a decrease in electronegativity (the ability to attract electrons) of elements and an increase in reducing properties (the ability to donate electrons to other atoms in chemical reactions).
Typical
metals are s-elements (elements of the IA group from Li to Fr. elements of the PA group from Mg to Ra). The general electronic formula of their atoms is ns1-2. They are characterized by oxidation states + I and + II, respectively.
The small number of electrons (1-2) in the outer energy level of typical metal atoms means that these electrons are easily lost and exhibit strong reducing properties, as reflected by low electronegativity values. This implies the limited chemical properties and methods of obtaining typical metals.
A characteristic feature of typical metals is the tendency of their atoms to form cations and ionic chemical bonds with non-metal atoms. Compounds of typical metals with non-metals are ionic crystals “metal cation-non-metal anion”, for example K+ Br–, Ca2+ O2-. Cations of typical metals are also included in compounds with complex anions - hydroxides and salts, for example Mg2+(OH–)2, (Li+)2СО32-.
The A-group metals that form the amphoteric diagonal in the Periodic Table Be-Al-Ge-Sb-Po, as well as the metals adjacent to them (Ga, In, Tl, Sn, Pb, Bi) do not exhibit typical metallic properties. The general electronic formula of their atoms is ns 2 np 0-4
implies a greater variety of oxidation states, a greater ability to retain their own electrons, a gradual decrease in their reducing ability and the appearance of oxidizing ability, especially in high oxidation states (typical examples are compounds Tl III, PbIV, Biv). Similar chemical behavior is characteristic of the majority of (d-elements, i.e. elements of the B-groups of the Periodic Table (typical examples are the amphoteric elements Cr and Zn).
This manifestation of duality (amphoteric) properties, both metallic (basic) and non-metallic, is due to the nature of the chemical bond. In the solid state, compounds of atypical metals with nonmetals contain predominantly covalent bonds (but less strong than bonds between nonmetals). In solution, these bonds are easily broken, and the compounds dissociate into ions (in whole or in part). For example, the metal gallium consists of Ga2 molecules; in the solid state, the chlorides of aluminum and mercury (II) AlCl3 and HgCl2 contain strongly covalent bonds, but in solution AlCl3 dissociates almost completely, and HgCl2 - to a very small extent (and even then into the ions HgCl+ and Сl–).