Global production of primary and secondary refined copper is currently around 15-16 million tonnes per year. Half of all copper produced is used in the electrical industry. The bulk of copper is obtained from primary raw materials, but a very significant proportion of copper is also produced from secondary raw materials. Thus, M1 sheet and electrical copper tapes, copper rods, and wire are produced mainly from primary raw materials; from the secondary - copper tube M2, again wire, roofing copper.
Natural compounds containing copper
Pure copper, which is what its nuggets represent, is found in nature in very small quantities. Copper is mainly found in nature in the form of various compounds, the most common of which are the following.
- Bornite is a mineral that got its name in honor of the Czech scientist I. Born. This is a sulfide ore, the chemical composition of which is characterized by its formula - Cu5FeS4. Bornite has other names: variegated pyrite, copper purple. In nature, this ore is presented in two polymorphic forms: low-temperature tetragonal-scalenohedral (temperature less than 228 degrees) and high-temperature cubic-hexaoctahedral (more than 228 degrees). This mineral can have different types depending on its origin. Thus, exogenous bornite is a secondary early sulfide, which is very unstable and is easily destroyed by weathering. The second type, endogenous bornite, is characterized by variable chemical composition, which may contain chalcocite, galena, sphalerite, pyrite and chalcopyrite. Theoretically, minerals of these types can contain from 25.5% sulfur, more than 11.2% iron and over 63.3% copper, but in practice this content of these elements is never maintained.
- Chalcopyrite is a mineral whose chemical composition is characterized by the formula CuFeS2. Chalcopyrite, which is of hydrothermal origin, was previously called copper pyrite. Along with sphalerite and galena, it is included in the category of polymetallic ores. This mineral, which, in addition to copper, contains iron and sulfur, is formed as a result of metamorphic processes and can be present in two types of copper ores: contact-metasomatic type (skarns) and mountain metasomatic (greisens).
- Chalcocite is a sulfide ore, the chemical composition of which is characterized by the formula Cu2S. This ore contains a significant amount of copper (79.8%) and sulfur (20.2%). This ore is often referred to as “copper lustre”, due to the fact that its surface appears as a shiny metal, ranging in shades from lead-gray to completely black. In copper-bearing ores, chalcocite appears as dense or fine-grained inclusions.
Chalcopyrite
In nature, there are also rarer minerals that contain copper.
- Cuprite (Cu2O), a member of the oxide group of minerals, can often be found in places where there is malachite and native copper.
- Covelline is a sulfide rock formed metasomatically. This mineral, whose copper content is 66.5%, was first discovered at the beginning of the last century in the vicinity of Vesuvius. Now covellite is actively mined in deposits in countries such as the USA, Serbia, Italy, and Chile.
- Malachite is a mineral well known to everyone as an ornamental stone. Surely everyone has seen products made from this beautiful mineral in the photo or even owns them. Malachite, which is very popular in Russia, is copper carbonate or copper dihydrocoxcarbonate, which belongs to the category of polymetallic copper-containing ores. The malachite found indicates that there are deposits of other minerals containing copper nearby. In our country, a large deposit of this mineral is located in the Nizhny Tagil region; previously it was mined in the Urals, but now its reserves there are significantly depleted and are not being developed.
- Azurite is a mineral that is also called “copper blue” due to its blue color. It is characterized by a hardness of 3.5–4 units; its main deposits are developed in Morocco, Namibia, Congo, England, Australia, France and Greece. Azurite is often intergrown with malachite and occurs in places where deposits of sulfide-type copper-bearing ores are located nearby.
Malachite
Natural minerals containing copper
Native copper is rarely found in nature. Much more often it is part of various minerals. Only a few of them are of industrial importance:
- chalcocite;
- bornite;
- chalcopyrite
Chalcocite is also found in Eurasia - in the Northern Urals, Kazakhstan, Spain, Germany - and in America - the USA, Chile. Bornite is found both in porphyry (igneous) rocks and in the zone of secondary sulfide enrichment. Found in deposits of Europe, America, Africa. However, the main mineral of copper is considered to be chalcopyrite. It contains up to 80% Cu. Present in all types of ores. It was even found in lunar soil.
Other, less common, but used in industrial production, minerals:
- cuprite;
- azurite;
- malachite;
- brochantite;
- chrysotile.
Most of them accompany each other and are adjacent to the fields.
Materials scientist
To obtain copper, copper ores are used (copper content - 1...6%), as well as waste of copper and its alloys.
Copper in nature is found in the form of sulfur compounds (CuS, Cu2S), oxides (CuO, Cu2O), hydrocarbonates (Cu(OH)2), carbon dioxide compounds (CuCO3) in sulfide ores and native copper metal.
The most common ores are copper pyrite and copper luster, containing 1...2% copper.
90% of primary copper is obtained by pyrometallurgical method, 10% by hydrometallurgical method.
The hydrometallurgical method is the production of copper by leaching it with a weak solution of sulfuric acid and subsequent separation of copper metal from the solution. The method is used when processing low-grade ores; it does not allow the extraction of precious metals along with copper.
The production of copper by the pyrometallurgical method consists of beneficiation, roasting, smelting for matte, purging in a converter, and refining.
Enrichment of copper ores is carried out by flotation and oxidative roasting.
The flotation method is based on the use of different wettability of copper-containing particles and waste rock. The essence of flotation is the selective adhesion of certain mineral particles suspended in an aqueous medium to the surface of air bubbles, with the help of which these mineral particles rise to the surface. The method allows you to obtain copper powder concentrate containing 10...35% copper.
Copper ores and concentrates containing large amounts of sulfur are subjected to oxidative roasting. In the process of heating the concentrate or ore to 700...800 0C in the presence of atmospheric oxygen, sulfides are oxidized and the sulfur content is reduced by almost half the original value. Only poor concentrates (with a copper content of 8...25%) are fired, and rich (25...35% copper) concentrates are melted without firing.
After roasting, the ore and copper concentrate are smelted into matte, which is an alloy containing copper and iron sulfides (Cu2S, FeS). The matte contains 20...50% copper, 20...40% iron, 22...25% sulfur, about 8% oxygen and admixtures of nickel, zinc, lead, gold, and silver. Depending on the chemical composition of the ore and its physical state, matte is produced either in shaft furnaces, if the raw material is lump copper ore containing a lot of sulfur, or in reverberatory furnaces, if the starting product is powdered flotation concentrate. Most often, smelting is carried out in fiery reverberatory furnaces. The temperature in the smelting zone is 1450 0C.
The resulting copper matte, in order to oxidize sulfides and iron, is subjected to blowing with compressed air in horizontal converters with side blast. The resulting oxides are converted into slag, and sulfur into SO2. Heat in the converter is released due to chemical reactions without fuel supply. The temperature in the converter is 1200…1300 ºC. Thus, the converter produces blister copper containing 98.4...99.4% copper, 0.01...0.04% iron, 0.02...0.1% sulfur and a small amount of nickel, tin, antimony, silver, gold. This copper is poured into a ladle and poured into steel molds or a casting machine.
Blister copper is refined to remove harmful impurities, followed by fire and then electrolytic refining.
The essence of fire refining of blister copper is the oxidation of impurities that have a greater affinity for oxygen than copper, removing them with gases and converting them into slag. After fire refining, copper with a purity of 99...99.5% is obtained. It is poured into molds and ingots are obtained for further smelting of alloys (bronze and brass) or ingots for electrolytic refining.
Electrolytic refining is carried out to obtain copper free of impurities (99.95% Cu).
Electrolysis is carried out in baths where the anode is made of fire-refined copper, and the cathode is made of thin sheets of pure copper. The electrolyte is an aqueous solution of CuSO4 (10...16%) and H2SO4 (10...16%).
When a direct current is passed, the anode dissolves, the copper goes into solution, and copper ions are discharged at the cathodes, depositing a layer of pure copper on them.
Impurities are deposited at the bottom of the bath in the form of sludge, which is processed to extract metals: silver, antimony, selenium, tellurium, gold, etc...
The cathodes are unloaded after 5...12 days, when their mass reaches 60...90 kg. They are thoroughly washed and then melted in electric furnaces.
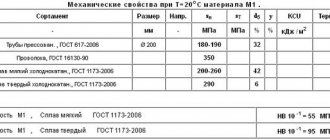
Copper by purity is divided into grades: M0 (99.95% Cu), M1 (99.9%), M2 (99.7%), M3 (99.5%), M4 (99%).
Metal production (help on the Internet) copper Methods for producing copper
To obtain copper, pyro-, hydro- and electrometallurgical processes are used.
Pyrometallurgical process
obtaining copper from sulfide ores such as CuFeS2 is expressed by the overall equation:
2CuFeS2 + 5O2 + 2SiO2 = 2Cu + 2FeSiO3 + 4SO2.
Hydrometallurgical methods
copper production is based on the selective dissolution of copper minerals in dilute solutions of sulfuric acid or ammonia, from the resulting solutions copper is replaced by metallic iron:
CuSO4+ Fe = Cu + FeSO4.
Electrolysis
get pure copper:
2CuSO4 + 2H2O 2Cu + O2 + 2H2SO4;
Copper is released at the cathode, oxygen is released at the anode.
Physical and chemical properties:
The crystal lattice of metallic copper is cubic face-centered, lattice parameter a = 0.36150 nm. Density 8.92 g/cm3, melting point 1083.4°C, boiling point 2567°C. Among all other metals, copper has one of the highest thermal conductivities and one of the lowest electrical resistances (at 20°C, resistivity 1.68 × 10–3 ohm m).
Finding in nature:
in the earth's crust the copper content is about 5·10–3% by mass.
Copper is very rarely found in native form (the largest nugget, 420 tons, was found in North America). Of the ores, the most widespread are sulfide ores: chalcopyrite, or copper pyrite, CuFeS 2 (30% copper), covellite CuS (64.4% copper), chalcocite, or copper luster, Cu2S (79.8% copper), bornite Cu5FeS4 ( 52-65% copper).
There are also many copper oxide ores, for example: cuprite Cu2O (81.8% copper), malachite CuCO3·Cu(OH)2 (57.4% copper) and others. There are 170 known copper-containing minerals, of which 17 are used on an industrial scale. There are many different copper ores, but there are few rich deposits on the globe, and besides, copper ores have been mined for many hundreds of years, so some deposits have been completely exhausted. Often the source of copper is polymetallic ores, which, in addition to copper, contain iron (Fe), zinc (Zn), lead (Pb), and other metals. As impurities, copper ores usually contain trace elements (cadmium, selenium, tellurium, gallium, germanium and others), as well as silver and sometimes gold. For industrial development, ores are used in which the copper content is slightly more than 1% by weight, or even less. Sea water contains approximately 1 10
-8% copper.
Receipt:
Industrial copper production is a complex multi-stage process.
The mined ore is crushed, and flotation beneficiation is usually used to separate the waste rock. The resulting concentrate (contains 18-45% copper by weight) is fired in an air-blast furnace. As a result of firing, cinder is formed - a solid substance containing, in addition to copper, also impurities of other metals. The cinder is melted in reverberatory furnaces or electric furnaces. After this smelting, in addition to slag, a so-called matte is formed, in which the copper content is up to 40-50%. Next, the matte is converted - compressed air enriched with oxygen is blown through the molten matte. Quartz flux (SiO 2 sand) is added to the matte.
During the conversion process, iron sulfide FeS contained in the matte as an undesirable impurity passes into slag and is released in the form of sulfur dioxide SO2: 2FeS + 3O2 + 2SiO2 = 2FeSiO3 + 2SO2
At the same time, copper (I) sulfide Cu2S is oxidized:
2Cu2S + 3O2 = 2Cu2O + 2SO2
The Cu2O formed at this stage further reacts with Cu2S:
2Cu2O + Cu2S = 6Cu + SO2
As a result, the so-called blister copper appears, in which the content of copper itself is already 98.5-99.3% by weight. Next, the blister copper is subjected to refining. Refining at the first stage is fire refining; it involves melting blister copper and passing oxygen through the melt. Impurities of more active metals contained in blister copper actively react with oxygen and turn into oxide slag. At the final stage, the copper is subjected to electrochemical refining in a sulfuric acid solution, with blister copper serving as the anode and purified copper being separated at the cathode. During such purification, impurities of less active metals present in the blister copper precipitate in the form of sludge, and impurities of more active metals remain in the electrolyte. The purity of refined (cathode) copper reaches 99.9% or more.
studfiles.net
Technology
Bessemerizing is an iron smelting process that produces steel of relatively high quality. It should be noted that such technology is used extremely rarely today. This is due to the emergence of quite a large number of modern technologies that make it possible to obtain higher quality steel in less time.
The entire Bessemer steel production process can be divided into several main stages:
- Cast iron is poured into the converter through the neck. An important point is that in this position the device must be in a horizontal position, since there is a possibility that the nozzle will be filled with metal. Nozzles are necessary to blow through the charge. It is the oxidation of impurities and their removal as slag that makes it possible to obtain steel of improved quality.
- The next stage is to start the blast and turn the converter into a vertical position.
- In order to ensure the oxidation of harmful impurities and excess carbon, the metal is blown with air. At this stage, slag is formed, with which unnecessary chemicals are removed.
- After a sufficiently long period of purging, the converter is turned over again into a horizontal position, and the purging of molten metal stops.
- The molten metal is drained into a ladle and deoxidized by adding special substances.
At the time of the start of purging the composition, active oxidation of manganese and silicon occurs. At the initial stage, carbon is practically not oxidized. This is due to the fact that this component reacts exclusively to exposure to high temperatures. In addition, the process of oxidation of impurities is influenced by thermodynamic factors that determine the activity of oxygen transfer to the sites of the Bessemer process.
Considering this technology, we note the following points:
- At the first stage, a large number of different slags are formed, which contains a high concentration of silica. The time interval for the first stage is 2-5 minutes.
- The second stage of the Bessemer production process provides the most favorable conditions for carbon oxidation. An example is an increase in operating temperature to approximately 2000 degrees Celsius. The duration of this stage is approximately 13 minutes. At the end of this stage, the temperature drops to approximately 1600 degrees Celsius.
- High quality steel can be achieved using various bessemerization methods. It all depends on the characteristics of the composition of the scrap used and the concentration of the cream in the composition.
- In order to eliminate the possibility of metal overblowing, the active air supply is stopped already at the second stage.
- Only at the third stage can active oxidation of iron be noted, which causes the release of brown smoke. This stage begins at the moment when the carbon concentration is less than 0.1%.
As previously noted, the Bessemer method of steel production has become widespread due to its high productivity. Foundries quite often install equipment that has a charge of about 35 tons.
Bessemer method of steel smelting
Today, the Bessemer method of steel production is practically not used, which is due to the low quality of the resulting metal and its fairly high cost.
Source
Features of copper ores
Copper-containing ores are characterized as multi-element. The most common connections are with:
- iron;
- gray;
- copper.
The following may be present in minor concentrations:
- nickel;
- gold;
- platinum;
- silver.
Deposits all over the world have approximately the same set of chemical elements in the ore composition; they differ only in their percentages. To obtain pure metal, various industrial methods are used. Almost 90% of metallurgical enterprises use the same method for producing pure copper - pyrometallurgical.
One of the largest ore mines produces 17 million tons of copper per year
The design of this process also makes it possible to obtain metal from recycled materials, which is a significant advantage for industry. Since the deposits belong to the group of non-renewable deposits, reserves decrease every year, ores become poorer, and their extraction and production becomes expensive. This ultimately affects the price of the metal on the international market. In addition to the pyrometallurgical method, there are other methods:
- hydrometallurgical;
- fire refining method.
Copper production
After mining the ore, the next problem arises: how to extract the necessary material from it? There are several ways.
One of the oldest technologies involved burning malachite ores with limited air access. The mass placed in pots, mixed with coal, burned, releasing carbon monoxide. Which led to achieving the desired result - obtaining copper that was sufficiently pure for its time.
It is clear that over the past centuries, methods and methods of ore processing have undergone major changes, driven by the goal of achieving the most optimal results for any type of primary raw material. That is why modern metallurgy is based on three main methods for producing copper.
Pyrometallurgical method
Based on high-temperature processes, the pyrometallurgical method is ideally suited for sulfide ores, which are sometimes quite poor in copper concentration. It allows you to extract metal even with a content of 0.5%.
But first of all, the feedstock is enriched during the flotation process. Its essence lies in thoroughly grinding the ore, filling it with water, and adding complex organic flotation reagents. They envelop mineral particles containing copper alloys, giving them non-wetting.
At the second stage of this process, foam is created in the solution, the bubbles of which pick up particles coated with organic matter. This happens under the influence of air flow, as a result of which the formations float to the surface, from where they are later taken up. The foam saturated with copper compounds is collected, squeezed out and dried.
After which the resulting concentrate is fired at a temperature of 14,000 C. This is necessary to remove sulfur and oxidize sulfides. Then high-temperature (14,0000 - 15,0000C) smelting is carried out in shaft furnaces to produce an alloy of iron and copper - matte. Next, in the process of Bessemer smelting in a converter under the influence of oxygen, the oxide is obtained, and then the blister copper itself, containing 90.95% of the metal. In this case, sulfur turns into an acid residue, and iron into silicate slag.
You can obtain pure copper from a rough substance using:
- fire refining,
- electrolysis,
- exothermic reduction reaction under the influence of hydrogen.
Hydrometallurgical method
To extract copper and a number of other metals from polymetallic ores containing less than 0.5% of the desired mineral, the hydrometallurgical method is used.
The extracted minerals are dissolved using non-concentrated sulfuric acid or ammonia. Copper is obtained from the resulting liquids during the displacement reaction. Metallic iron is used to carry out the reaction.
Electrolysis method
The method is designed to obtain pure copper through an electrolytic reaction.
Its technology is to produce pure copper thin sheet cathodes and thick plate anodes from blister copper. Placed then in a bath filled with copper sulfate, they react under the influence of electric current. Copper dissolves on the anodes and is deposited on the cathodes. Released impurities are removed by chemical methods.
Copper pipes
§ 12. Obtaining metals -
1. In the work of the German scientist in the field of metallurgy and physician G. Agricola (16th century) “12 books on metals” it is said: “By subjecting the ore to heating, roasting and calcination, this removes part of the substances mixed with the metal...” and further “... smelting is necessary, since only through it are rocks and solidified juices (brines) separated from metals, which acquire their characteristic color, are purified and become useful to man in many ways.”
What types of metallurgy did Agricola write about? Illustrate his statement with examples of chemical reaction equations. G. Afikol had pyrometallurgy in mind in his book.
Pyrometallurgical processes involve roasting, in which metal compounds contained in ores, in particular sulfides, are converted into oxides, and sulfur is removed in the form of SO2, for example: 2. Which method of obtaining copper - using sulfuric acid or bacterial - is environmentally safer?
The bacterial method of producing copper is environmentally safer.
3. Why can’t alkali and alkaline earth metals be obtained by the hydrometallurgical method?
Alkali and alkaline earth metals cannot be obtained by the hydrometallurgical method, because this method is based on the separation of metals from solution under the influence of an electric current.
And if we isolate an alkali metal in its pure form from a solution, it will immediately react with water, forming a hydroxide. 4. Propose a technological chain for the production of lead from the galena mineral PbS. Write down the reaction equations. 5. How can iron and sulfuric acid be obtained from FeS2 pyrite? Write down the reaction equations. 6. How many kilograms of copper are obtained from 120 tons of enriched rock containing 20% copper (I) sulfide if the copper yield is 90% of the theoretically possible?
gdz-himiya.ru
Varieties of copper ores
There are nine geological types of copper ores of industrial importance:
- Iron-nickel ores occurring in igneous rocks.
- Cuprous sandstones and shales. Stratiform reserves account for 30% of copper reserves and therefore occupy second place in this list.
- Copper-nickel. The deposits are distinguished by a variety of shapes with large inclusions of the desired metal.
- Porphyry copper. They are the undisputed leader and provide 40% of global copper production.
- Carbonatite. They are unique in that there is only one deposit in the world; in addition, they contain alkaline compounds.
- Quartz-sulfide. They do not play a significant role in ensuring production.
- Native. They are located in oxidation areas of copper-sulfide ore mines.
- Skarn. They are located among limestones and are characterized by extreme heterogeneity of morphological structure.
Copper in the listed list of ores can be presented in sulfide, oxide or mixed form, which determines the corresponding types of deposits. Based on their structure in rocks, deposits are divided into disseminated, massive and continuous textures. In the near future, this list may be supplemented by ores lying at the bottom of seas and oceans, as well as nodules of uranium deposits.
Copper mining countries
The leading positions in global copper production (2022 data in quantitative terms of the metal mined per year) are occupied by:
- Chile – 5.8 million tons.
- Peru – 2.4 million tons.
- China – 1.6 million tons.
- USA – 1.2 million tons.
- Congo - 1.2 million tons.
Judging by expert estimates, the total volume of as yet unexplored copper reserves in the world is 3.5 billion tons. These reserves should be enough for the next century and a half.
Author: Yuri Florinskikh All articles by this author
Latest articles by the author: The largest producers of milk and dairy products in the world Diamonds: properties, mining methods and applications
Fire refining technology for blister copper
This method of obtaining pure copper is used when the starting material is copper scrap.
The process takes place in special reverberatory furnaces, which are fired by coal or oil. The melted mass fills the bath, into which air is blown through iron pipes:
- pipe diameter – up to 19 mm;
- air pressure – up to 2.5 atm;
- oven capacity – up to 250 kg.
During the refining process, copper raw materials are oxidized, sulfur burns out, then metals. Oxides do not dissolve in liquid copper, but float to the surface. To remove them, quartz is used, which is placed in the bath before the refining process begins and is placed along the walls.
If the scrap metal contains nickel, arsenic or antimony, the technology becomes more complicated. The percentage of nickel in refined copper can only be reduced to 0.35%. But if other components are present (arsenic and antimony), then nickel “mica” is formed, which dissolves in copper and cannot be removed.
Source
Stages of pyrometallurgical copper production
General methods for obtaining metal from ore
Industrial copper production using the pyrometallurgical method has advantages over other methods:
- the technology provides high productivity - it can be used to produce metal from rocks in which the copper content is even lower than 0.5%;
- allows you to efficiently process secondary raw materials;
- a high degree of mechanization and automation of all stages has been achieved;
- its use significantly reduces emissions of harmful substances into the atmosphere;
- The method is economical and effective.
Enrichment
Ore beneficiation scheme
At the first stage of production, it is necessary to prepare the ore, which is delivered to processing plants directly from the quarry or mine. Often there are large pieces of rock that must first be crushed.
This happens in huge crushing units. After crushing, a homogeneous mass is obtained, with a fraction of up to 150 mm. Pre-enrichment technology:
- raw materials are poured into a large container and filled with water;
- oxygen is then added under pressure to form foam;
- metal particles stick to the bubbles and rise to the top, and waste rock settles at the bottom;
- Next, the copper concentrate is sent for roasting.
Burning
This stage aims to reduce the sulfur content as much as possible. The ore mass is placed in a furnace where the temperature is set at 700–800
O
C. As a result of thermal exposure, the sulfur content is halved. Sulfur oxidizes and evaporates, and some of the impurities (iron and other metals) pass into an easily slag state, which will facilitate later smelting.
Roasting ore to reduce sulfur levels
This stage can be omitted if the rock is rich and contains 25–35% copper after enrichment; it is used only for low-grade ores.
Melting for matte
The matte smelting technology makes it possible to obtain blister copper, which varies by grade: from MCh1 - the purest to MCh6 (contains up to 96% pure metal). During the smelting process, the raw material is immersed in a special furnace in which the temperature rises to 1450
O
WITH.
Copper ore processing technology and black copper production
After the mass is melted, it is purged with compressed oxygen in converters. They have a horizontal appearance, and the blowing is carried out through a side hole. As a result of blowing, iron and sulfur sulfides are oxidized and converted into slag. Heat in the converter is generated due to the flow of hot mass; it does not heat up additionally. The temperature is 1300
O
WITH.
General scheme of copper smelting
At the output of the converter, a rough composition is obtained, which contains up to 0.04% iron and 0.1% sulfur, as well as up to 0.5% other metals:
- tin;
- antimony;
- gold;
- nickel;
- silver
This rough metal is cast into ingots weighing up to 1200 kg. This is the so-called anode copper. Many manufacturers stop at this stage and sell such ingots. But since copper production is often accompanied by the extraction of precious metals contained in the ore, processing plants use the technology of refining the rough alloy. In this case, other metals are released and preserved.
Refining using copper cathode
The technology for producing refined copper is quite simple. Its principle is even used to clean copper coins from oxides at home. The production scheme looks like this:
Refined copper ingots
- the rough ingot is placed in a bath with electrolyte;
- a solution with the following content is used as an electrolyte: copper sulfate – up to 200 g/l;
- sulfuric acid – 135–200 g/l;
- colloidal additives (thiourea, wood glue) – up to 60 g/l;
- water.
O
WITH;
The entire electrolysis process takes place within 20–28 days. During this period, the copper cathode is removed up to 3–4 times. The weight of the plates is up to 150 kg.
How it's done: copper mining
During the refining process, dendrites can form on cathode copper - growths that reduce the distance to the anode. As a result, the speed and efficiency of the reaction decreases. Therefore, when dendrites appear, they are immediately removed.
Answers to exercises § 7. Chemistry 9th grade.
| Exercise 1 In the work of the German scientist in the field of metallurgy and physician G. Agricola (16th century) “12 books on metals” it is said: “By subjecting the ore to heating, roasting and calcination, this removes part of the substances mixed with the metal...” and further “... smelting is necessary , since only through it are rocks and hardened juices (brines) separated from metals, which acquire their characteristic color, are purified and become useful to man in many ways.” What types of metallurgy did Agricola write about? Illustrate his statement with examples of chemical reaction equations. Pyrometallurgy roasting: 2CuS + 3O2 = 2CuO + 2SO2 smelting: 2CuO + C = 2Cu + CO2 | Exercise 2 Which method of producing copper - using sulfuric acid or bacterial - is more environmentally friendly? The bacterial method is safer from an environmental point of view, since the process of obtaining copper does not use aggressive, harmful substances and therefore produces less waste harmful to the environment | Exercise: 3 Why can’t alkali and alkaline earth metals be obtained by the hydrometallurgical method? The hydrometallurgical method is the recovery of metals from aqueous solutions of their compounds (soluble salts of these metals). Since alkali and alkaline earth metals interact with water, it is impossible to obtain them from an aqueous solution of their salt; they react with water. | Exercise: 4 Propose a technological chain for the production of lead from the galena mineral PbS. Write down the reaction equations. PbS → PbO → Pb 2PbS + O2 = 2PbO + 3SO2 PbO + Al = Pb + Al2O3 |
reshebnikxim.narod.ru
Features of copper: its composition, structure and production technology
Copper, which belongs to non-ferrous metals, has been known for a long time. Its production was invented before people began to make iron.
According to assumptions, its active use occurred as a result of its availability and fairly simple extraction from copper-containing compounds and alloys.
So, let's look today at the properties and composition of copper, the world's leading countries in copper production, the manufacture of products from it and the features of these areas.
Copper has a high coefficient of electrical conductivity, which has increased its value as an electrical material. If previously up to half of all copper produced in the world was spent on electrical wires, now aluminum is used for these purposes as a more affordable metal. And copper itself becomes the most scarce non-ferrous metal.
This video discusses the chemical composition of copper:
The structural composition of copper includes many crystals: nickel, gold, calcium, silver, lead and many others. All metals included in its structure are distinguished by relative softness, ductility and ease of processing. Most of these crystals, when combined with copper, form solid solutions with continuous rows.
The unit cell of this metal is cubic in shape. For each such cell there are four atoms located at the vertices and the central part of the face.
Chemical composition
The composition of copper during its production may include a number of impurities that affect the structure and characteristics of the final product. At the same time, their content should be regulated both by individual elements and by their total quantity. Impurities that are found in copper include:
- Bismuth. This component negatively affects both the technological and mechanical properties of the metal. That is why it should not exceed 0.001% of the finished composition.
- Oxygen. It is considered the most undesirable impurity in copper. Its maximum content in the alloy is up to 0.008% and rapidly decreases when exposed to high temperatures. Oxygen negatively affects the ductility of the metal, as well as its resistance to corrosion.
- Manganese. In the case of the manufacture of conductive copper, this component negatively affects its conductivity. Already at room temperature it quickly dissolves in copper.
- Arsenic. This component creates a solid solution with copper and has virtually no effect on its properties. Its action is largely aimed at neutralizing the negative effects of antimony, bismuth and oxygen.
- Nickel. Forms a solid solution with copper and at the same time reduces its thermal and electrical conductivity.
- Tin. Creates a solid solution and enhances thermal conductivity.
- Selenium, sulfur. These two components have the same effect on the final product. They form a fragile connection with copper and amount to no more than 0.001%. As the concentration increases, the degree of ductility of copper sharply decreases.
- Antimony. This component is highly soluble in copper and therefore has minimal impact on its final properties. It is allowed no more than 0.05% of the total volume.
- Phosphorus. Serves as the main deoxidizer of copper, the maximum solubility of which is 1.7% at a temperature of 714°C. Phosphorus, in combination with copper, not only promotes better welding, but also improves its mechanical properties.
- Zinc. Contained in a small amount of copper, it has virtually no effect on its thermal and electrical conductivity.
The process and proper sequence of copper production will be discussed next.
Areas of use
Copper is in greatest demand in electrical engineering. Due to its low electrical resistance, it is actively used for the manufacture of wires, cables, conductive elements of transformers, and electric motor windings are made from it. More than half of all mined copper is consumed by the industry.
The metal has good strength and ductility, which makes it suitable for the manufacture of products of complex shapes: household items, jewelry; for creating seamless pipes. Copper pipes are often used in equipment and various engineering systems to organize heat exchange. The properties of copper such as low coefficient of thermal expansion and high thermal conductivity are used here.
Copper alloys are widely used:
- bronze - a compound with tin, the name is also used for alloys of copper with other metals, such as aluminum, lead, silicon, beryllium and others;
- brass - with zinc;
- cupronickel, nickel silver, manganin - with nickel;
Copper is also included as one of the components in other compounds, for example, in duralumin. Jewelers often use copper alloys with the addition of gold.