Characteristics of magnesium
Industrial production and use of magnesium began relatively recently - only about 100 years ago. This metal has a low mass, as it has a relatively low density (1.74 g/cmᶟ), good stability in air, alkalis, gaseous environments containing fluorine and in mineral oils.
Its melting point is 650 degrees. It is characterized by high chemical activity up to spontaneous combustion in air. The tensile strength of pure magnesium is 190 MPa, the elastic modulus is 4,500 MPa, and the relative elongation is 18%. The metal has a high damping capacity (effectively absorbs elastic vibrations), which provides it with excellent tolerance to shock loads and reduced sensitivity to resonance phenomena.
Other features of this element include good thermal conductivity, low ability to absorb thermal neutrons and interact with nuclear fuel. Thanks to the combination of these properties, magnesium is an ideal material for creating hermetically sealed shells for high-temperature elements of nuclear reactors.
Magnesium alloys well with various metals and is one of the strong reducing agents, without which the process of metallothermy is impossible.
In its pure form, it is mainly used as an alloying additive in alloys with aluminum, titanium and some other chemical elements. In ferrous metallurgy, with the help of magnesium, deep desulfurization of steel and cast iron is carried out, and the properties of the latter are also improved through spheroidization of graphite.
What is it like, “hot-tempered metal”
Our hero is an element of the second group of Mendeleev’s periodic table. Latin name Magnesium, atomic number 12.
Magnesium is now classified as an alkaline earth metal. However, it was not previously considered as such - its hydroxide is not an alkali, although the solution in the presence of phenolphthalein (an indicator) turns a faint pink color. High-grade alkalis with phenolphthalein are painted in a thick crimson color.
Pure magnesium has a close-packed hexagonal crystal structure.
The structure of the atom indicates that it belongs to metals. The electronic formula of the element is 1s 2 2s 2 2p 6 3s 2. That is, at the external energy level of magnesium there is a pair of electrons dangling, ready at any moment to “fall to the left” - to react with another element. Some people still remember that the property of metals at the outer level is to have from 1 to 3 electrons.
Magnesium and alloying additives
The most common alloying additives used in magnesium-based alloys include elements such as aluminum, manganese and zinc. Aluminum improves the structure, increasing the fluidity and strength of the material. The introduction of zinc also makes it possible to obtain stronger alloys with reduced grain size. With the help of manganese or zirconium, the corrosion resistance of magnesium alloys is increased.
The addition of zinc and zirconium provides increased strength and ductility of metal mixtures. And the presence of certain rare earth elements, for example, neodymium, cerium, yttrium, etc., helps to significantly increase the heat resistance and maximize the mechanical properties of magnesium alloys.
To create ultra-light materials with a density of 1.3 to 1.6 g/mᶟ, lithium is introduced into alloys. This additive makes it possible to reduce their weight by half compared to aluminum metal mixtures. At the same time, their indicators of plasticity, fluidity, elasticity and manufacturability reach a higher level.
Manufacturing
Hot and cold work
Magnesium alloys harden quickly under any type of cold working and therefore cannot be subjected to severe cold deformation without reworking. annealing. Sharp bending, rotating, or pulling must be done at temperatures between 260 and 316 °C (500 and 600 °F), although gentle bending around large radii can be done when cold. Slow molding gives better results than fast molding. Press forging is preferred to hammer forging because the press allows the metal to flow longer. The range of plastic forging is 500 to 800 °F (260 to 427 °C). Metal processed outside this range breaks easily.
Casting
Magnesium alloys, especially dispersion-hard ones, are used in casting. Sand casting, permanent mold and injection molding techniques are used, but plaster of Paris casting has not yet been perfected. Sand casting requires a special technique because magnesium reacts with moisture in the sand to form magnesium oxide and release hydrogen. The oxide forms blackened areas called burns on the surface of the casting, and the hydrogen released can cause porosity. Inhibitors such as sulfur, boric acid, ethylene glycol or ammonium fluoride are mixed with wet sand to prevent the reaction. All gravity-fed molds require a very high column of molten metal so that the pressure is great enough to force gas bubbles out of the casting and force the metal to grip the mold part. Casting wall thickness should be at least 5/32 inch in most conditions. Very large fillets must be provided at all reentrant corners since stress concentrations in magnesium castings are particularly dangerous. Permanent mold castings are made from the same alloys and have approximately the same physical properties as sand castings. Since the solidification shrinkage of magnesium is about the same as that of aluminum, aluminum molds can often be adapted to produce magnesium alloy castings (although the valve may need to be replaced). Cold chamber injection moldings are used for mass production of small parts. The rapid solidification caused by the contact of the liquid metal with the cold matrix produces a casting with a dense structure and excellent physical properties. Finishing and dimensional accuracy are very good and machining is only necessary where maximum precision is required. Typically these castings are not heat treated.
Welding, soldering and riveting
Many standard magnesium alloys are easy to machine. welded with gas or resistance welding equipment, but cannot be cut with an oxy-fuel torch. Magnesium alloys are not welded with other metals because brittle intermetallic compounds may form or because the combination of metals may promote corrosion. If two or more parts are welded, their composition must be the same. Soldering of magnesium alloys is possible only for sealing surface defects of parts. Solders are even more corrosive than aluminum and the parts should never be able to withstand stress. Riveted Connections in magnesium alloy structures typically use aluminum or aluminum-magnesium alloy rivets. Magnesium rivets are not often used because they must be driven in while hot. Rivet holes should be drilled, especially in thick sheets and extruded profiles, as stamping tends to produce rough hole edges and stress concentrations.
Treatment
The special attractiveness of magnesium alloys lies in their unusually good characteristics. mechanical processing Their properties surpass even threaded brass. The power required to cut them is low, and extremely high speeds (5,000 feet per minute in some cases) can be used. The best cutting tools have a special shape, but tools for cutting other metals can be used, although their efficiency is slightly lower. When cutting magnesium at high speed, tools must be sharp and must always cut. A dull, dragging tool operating at high speed can generate enough heat to ignite fine chips. Since chips and dust from grinding can pose a fire hazard, grinding should be carried out with coolant or using a dust concentration device under water. A magnesium grinder should also not be used on ferrous metals, as a spark could ignite accumulated dust. If magnesium catches fire, it can be extinguished with cast iron shavings, dry sand or other materials specially prepared for this purpose. Water or liquid fire extinguishers should never be used because they may spread the fire. In fact, magnesium chips and dust are much more difficult to ignite than is usually assumed, and for this reason they do not present much difficulty in handling. The special methods that must be used in the production of magnesium (processing, casting and joining) significantly increase the cost of production. When choosing between aluminum and magnesium or a given part, the base cost of the metal may not give much advantage to either, but typically manufacturing operations make magnesium more expensive.[1] There is perhaps no group of alloys where extrusion is more important than these, since the comparatively coarse grain structure of the cast material makes most of them too susceptible to cracking to be worked by other means until sufficient deformation has been achieved. for cleaning grain. Therefore, with the exception of one or two soft alloys, machining is always a preliminary step to other forming processes.
Hot extrusion
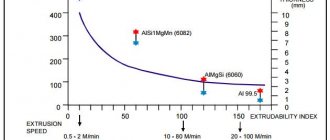
Pure magnesium is not enough. extruded because it has somewhat poor properties, especially regarding its yield strength. Currently, the main focus is on alloying elements: aluminum, zinc, cerium and zirconium; Manganese is usually also present because although it has little effect on strength, it plays an important role in improving corrosion resistance. One important binary alloy, containing up to 2.0% manganese, is widely used for sheet metal production. It is relatively soft and easier to extrude than other alloys, and is also one of the few that can be directly rolled without pre-extrusion. In the UK, extrusions are made from 2.87–12 in (73–305 mm) diameter blanks. . On presses with a capacity of 600 to 3500 tons; Normal maximum pressure on the workpiece is 30-50 t/sq.m. In the US, the chemical company Dow recently installed a 13,200 ton press capable of processing workpieces up to 32 inches. The extrusion technique is generally similar to that for aluminum-based alloys, but Wilkinson and Fox say die design requires special attention, and they believe the bearings must be short and have sharp die entries. Pipe extrusion of AM503, ZW2 and ZW3 alloys is now done using bridge dies. (Aluminum alloys do not weld well.) In contrast to the previous practice of using drilled blanks, mandrel piercing is now used when extruding large diameter ZW3 alloy pipe.
The extrusion hardness of alloys increases in proportion to the number of reinforcing elements they contain, and the temperature used is usually higher the greater the number. The workpiece temperature is also affected by the size of the sections, being higher for heavy reductions but typically in the range of 250–450 °C (482–842 °F). The temperature of the container should match the temperature of the workpiece or only slightly exceed it. Preheating of the workpieces should be carried out uniformly to promote, as far as possible, a homogeneous structure by absorbing compounds such as Mg4Al present in the alloys.
Fox points out, and this also applies to aluminum alloys. The original structure of the workpiece is important, and casting methods that result in fine grains make sense. Coarse material contains larger particles of compounds that are less easily dissolved and tend to cause a solution gradient. In magnesium alloys this causes internal stress as the solution undergoes slight compression and can also affect the uniformity of response to subsequent heat treatment.
Binary magnesium-manganese alloy (AM505) is easily extruded at low pressures over a temperature range of 250 to 350 °C (482 to 662 °F). The actual temperature used depends on the reduction and length of the workpiece, not the desired properties. which are relatively insensitive to extrusion conditions. Good extrusion surface condition is achieved only at high speeds of the order of 50-100 feet per minute.
Alloys containing aluminum and zinc, and especially higher aluminum content alloys such as AZM and AZ855, have difficulty at high speeds due to heat resistance. Under conditions close to equilibrium, magnesium is capable of dissolving about 12 percent of aluminum, but in a cast state. Stocks of 4-5 percent usually represent the solubility limit. Consequently, alloys containing 6 percent Al or more contain Mg4Al3, which forms a melting eutectic at 435 C. Extrusion temperatures can range from 250 to 400 °C (482 to 752 °F), but at higher values rates are limited to approximately 12 feet per minute. Continuous casting improves the uniformity of these alloys, and water-cooling the dies or heating the cone of the blanks further facilitates their extrusion.
The introduction of magnesium-zinc-zirconium alloys, ZW2 and ZW3, represents a significant advance in magnesium alloy technology for a number of reasons. They have high strength, but because they do not contain aluminum, the casting contains only small amounts of the second phase. Because the solidus temperature is raised by approximately 100 °C (180 °F), the risk of hot shorts at relatively high extrusion speeds is greatly reduced. However, the mechanical properties are sensitive to preheating time of the preform, temperature and extrusion speed. Long preheat times, high temperatures and speeds provide properties similar to older aluminum alloys. Heat-up times must be short and temperatures and speeds must be low to obtain high performance. Increasing the zinc content to 5 or 6 percent, as in American alloy ZK60 and ZK61, reduces the sensitivity of mechanical properties to extrusion speed.
Alloying of zirconium-containing materials has been a major problem in their development. Zirconium is usually added from salt and careful control can give good results. Dominion Magnesium Limited in Canada has developed a traditional addition method via ligature.
The explanation for the low extrusion speeds required for successful extrusion of some magnesium alloys is not independent of the reasons put forward for other metals. Altwicker believes the most important reason is related. With a degree of recovery from crystal deformation that is less rivaled by rapid application of work, causing higher stresses and depleting the ability to slip in the crystals. This is noteworthy because the rate of recrystallization varies from one metal to another and with temperature. It is also a fact that metal processed in what is considered its operating range can often exhibit noticeable work hardening when quenched immediately after deformation - this shows that a temporary loss of ductility can easily accompany rapid processing.[10][full citation required
]
Casting alloys
This group includes alloys with the addition of magnesium, intended for the production of various parts and elements by shaped casting. They have different mechanical properties, depending on which they are divided into three classes:
- medium strength;
- high strength;
- heat resistant.
Based on their chemical composition, alloys are also divided into three groups:
- aluminum + magnesium + zinc;
- magnesium + zinc + zirconium;
- magnesium + rare earth elements + zirconium.
Magnesium versus aluminum, or why a Samsung Galaxy S7 made of magnesium would be amazing
It's that time of year again - the time of rumors, speculations, estimates and predictions regarding what the next Samsung Galaxy model will be. And the rumors surrounding the January announcement of the Samsung Galaxy S7 are already in full swing. How amazing will the phone be? Will it have radically improved performance? How will this be done? Will it be able to shoot laser beams and project holograms in the air? These questions are on many people’s minds right now. We will try to answer them with the help of our rich collection of rumors about the Samsung Galaxy S7.
Samsung Galaxy S7
Among the many rumors about the Galaxy S7, there is one that suggests that the next flagship from Samsung will be slightly different in terms of design. The new model will be made of glass and metal, like the Galaxy S6. But for the outer frame of the phone, magnesium alloy will be used instead of aluminum. The material will likely be used inside the device as part of its internal structure. If this turns out to be true, it will be absolutely amazing, and there are several reasons for this, which we will talk about next.
Contents of the article
So what is magnesium?
Magnesium is an alkaline earth metal with atomic number 12. It is a shiny gray solid with numerous properties and is often used when a shiny, durable material is required. However, magnesium itself is definitely not suitable for use in consumer products, as it is very reactive. We don't want our gadgets to easily corrode or suddenly catch fire, do we? This is why magnesium is mixed with other elements, including aluminum or zinc, to create the most practical alloys. For example, some premium laptops, digital cameras, and even some cell phones are made from magnesium alloy.
Parts made from this alloy are also used in aircraft, missiles, high-performance and other applications where weight reduction is important.
Magnesium
Why are magnesium alloys better than aluminum alloys?
There are several differences between magnesium alloys and aluminum alloys. Let's start with the fact that the first ones are easier. The body of the Galaxy S6, for example, is made of 6013 aluminum alloy, which has a density of 2.71 g/cm³ (0.0979 lb/in³). The density of the 7000-series aluminum used in the iPhone 6s is even higher. By comparison, magnesium alloys have a density of about 1.8 g/cm³ (0.065 lb/in³). These confusing numbers mean that magnesium alloys are about 33% lighter than their aluminum counterparts. This greatly affects the overall weight of the product we use. Most of us don't mind the lighter Galaxy S7, do we?
Although magnesium alloys are lighter, they are similar to (if not better than) aluminum alloys in terms of mechanical properties. They can be just as strong and durable. They also dissipate heat well. Such alloys withstand vibrations and shocks very well. They have less influence on the transmission of radio waves. In addition to all this, it is easier to make structural elements such as a phone case or frame from magnesium alloys, as they have favorable mechanical properties and a low melting point.
If magnesium alloys are so amazing, where have they been all this time?
The Samsung NX1 camera has a body made of magnesium alloy
Historically, aluminum has gained popularity faster, as the metal is great for everything from soda cans to car engines. It was light, strong, recyclable, and improved technology made it cheap. The commercial use of magnesium began much later, but the material's popularity is now on the rise as its cost-effectiveness approaches that of aluminum. On the one hand, magnesium raw materials are still much more expensive than aluminum, but on the other hand, it is easier for machines to make into an alloy, so it is cost-effective, just like aluminum.
Casting properties of alloys
Among the products of these three groups, aluminum-magnesium alloys have the best casting properties. They belong to the class of high-strength materials (up to 220 MPa), therefore they are the best option for the manufacture of parts for aircraft engines, cars and other equipment operating under mechanical and temperature loads.
To increase the strength characteristics, aluminum-magnesium alloys are alloyed with other elements. But the presence of iron and copper impurities is undesirable, since these elements have a negative effect on the weldability and corrosion resistance of alloys.
Cast magnesium alloys are prepared in various types of melting furnaces: in reflective furnaces, in crucible furnaces with gas, oil or electric heating, or in crucible induction installations.
To prevent combustion during the melting process and during casting, special fluxes and additives are used. Castings are produced by sand, plaster, shell, pressure and lost wax casting.
conclusions
The development of engineering and technology requires the use of new materials and alloys
This is especially important for those areas of industry and technology in which the mass of the product is limited. Such areas include aviation, space technology, automotive industry, nuclear technology
In these areas of technology, technical devices are created, the mass of which in many cases should be minimal. Therefore, it is necessary to use materials with high structural properties and low weight. Such substances include magnesium-based alloys. As discussed above, the production of such alloys is a rather complex technological task, requiring the use of inert gases, etc. Therefore, the development of magnesium-based alloys and improvement of their processing properties has a great future.
Wrought alloys
Compared to casting alloys, wrought magnesium alloys are characterized by greater strength, ductility and toughness. They are used to produce blanks using rolling, pressing and stamping methods. As a heat treatment of products, hardening is used at a temperature of 350-410 degrees, followed by random cooling without aging.
When heated, the plastic properties of such materials increase, so the processing of magnesium alloys is carried out using pressure and at high temperatures. Stamping is performed at 280-480 degrees under presses using closed dies. During cold rolling, frequent intermediate recrystallization anneals are carried out.
When welding magnesium alloys, the strength of the weld of the product can be reduced in the sections where back welding was performed due to the sensitivity of such materials to overheating.
Aluminum-Copper Systems
In a heat-strengthened state they exhibit good mechanical properties, but are susceptible to corrosion. Additional surface treatment of metal products is required to increase corrosion resistance. The AL-Cu system is additionally doped with:
- manganese, it reduces chemical activity;
- silicon, the component affects the eutectic point - equilibrium of the liquid and solid phases;
- iron to improve strength properties, the decrease in ductility and corrosion resistance is compensated by nickel;
- Nickel in combination with iron increases heat resistance and improves their ability to age.
- copper improves the ability to deform and harden semi-finished and finished products at low temperatures. AL-Cu-Si (alcusine) alloys have anti-friction properties and are used in the manufacture of engine parts, transmissions, and vehicle chassis.
Areas of application of alloys with the addition of magnesium
Through the methods of casting, deformation and heat treatment of alloys, various semi-finished products are produced - ingots, plates, profiles, sheets, forgings, etc. These blanks are used for the production of elements and parts of modern technical devices, where the priority role is played by the weight efficiency of structures (reduced mass) while maintaining their strength characteristics. Compared to aluminum, magnesium is 1.5 times lighter, and 4.5 times lighter than steel.
Currently, the use of magnesium alloys is widely practiced in aerospace, automotive, military and other industries, where their high cost (some brands contain quite expensive alloying elements) is justified from an economic point of view by the possibility of creating more durable, faster, more powerful and safe equipment , which can work effectively in extreme conditions, including when exposed to high temperatures.
Due to their high electrical potential, these alloys are an optimal material for creating protectors that provide electrochemical protection of steel structures, for example, automobile parts, underground structures, oil platforms, sea vessels, etc., from corrosion processes occurring under the influence of moisture, fresh and sea water.
Alloys with the addition of magnesium have also found application in various radio engineering systems, where they are used to make sound ducts for ultrasonic lines to delay electrical signals.
Being in nature
Clark magnesium - 1.95% (19.5 kg/t). This is one of the most common elements of the earth's crust. Large amounts of magnesium are found in seawater in the form of a salt solution. The main minerals with a high mass content of magnesium:
- sea water - (0.12-0.13%),
- carnallite - MgCl2 • KCl • 6H2O (8.7%),
- bischofite - MgCl2 • 6H2O (11.9%),
- kieserite - MgSO4 • H2O (17.6%),
- epsomite - MgSO4 • 7H2O (9.9%),
- kainite - KCl • MgSO4 • 3H2O (9.8%),
- magnesite - MgCO3 (28.7%),
- dolomite - CaCO3 MgCO3 (13.1%),
- brucite - Mg(OH)2 (41.6%).
Magnesium salts are found in large quantities in salt deposits of self-sediment lakes. Deposits of carnallite of sedimentary origin are found in many countries.
Magnesite is formed predominantly in hydrothermal conditions and belongs to medium-temperature hydrothermal deposits. Dolomite is also an important magnesium raw material. Dolomite deposits are widespread and their reserves are enormous. They are genetically related to carbonate sedimentary layers and most of them are of Precambrian or Permian geological age. Dolomite deposits are formed by sedimentation, but can also arise when limestone is exposed to hydrothermal solutions, groundwater or surface water.
An extremely rare mineral is native magnesium, formed in flows of reducing gases and first discovered in 1991 in the coastal sediments of Chona (Eastern Siberia), and then in lavas in Southern Gissar (Tajikistan).