- Reports and messages
- Geography
- Metal alloys
A long time ago, people noticed, speaking about biology, for example, that if you combine two organisms, the third will surpass its parents.
It turns out that the same principle works in chemistry, so the history of the appearance of alloys can be considered straightforward. Someone simply noticed that when melted, metals are mixed and something new and more durable is obtained. According to the modern definition, an alloy is a chemical compound that must contain at least one metal, while the remaining components may not be metals.
For example, bronze, which is an alloy of copper + tin, is more durable, which is why people most often use alloys. Moreover, there are countless different variations of alloys, although at the moment only eighty-odd types of metals are known.
The alloys are also characterized by high resistance and hardness in addition to the fact that they have excellent casting properties. For example, tin bronze (copper + tin) lends itself better to casting than plain copper, so it is often used in sculpting works of art. Similar casting alloys also include cast iron (iron + carbon), duralumin (aluminum + copper + magnesium + manganese), etc.
Let's consider the classification of alloys:
- Compound. Alloys are divided according to their predominant components, for example: titanium, copper, nickel, etc.
- Value. Alloy names refer to the value of the components, for example: brass, tungsten steel, etc.
- Black metals. The name is most often used in metallurgical production. This group includes absolutely all alloys that contain iron.
- Non-ferrous metals. Again the name is used in metallurgy and refers to other metals besides iron.
Accordingly, the above groups are divided into subgroups with a narrower selection of common components or properties, for example.
Option No. 2
Alloys
1) Reasons for use 2) Classifications 3) Components and ligatures 4) Applications
Man took a revolutionary step when he realized that a mixture of copper and tin was much harder than any of these metals in their pure form. It is believed that this happened at least eight thousand years ago.
Tens of thousands of alloys are used in the modern world, and the development of new ones continues. Several criteria are used to classify alloys.
First of all, there are two large groups: ferrous metals (i.e. alloys based on iron) and non-ferrous metals (based on other elements).
Depending on where the metal will be used, it is classified as a general-purpose or special alloy. Further, binary and complex (ternary, quadruple, etc.) alloys are distinguished according to the number of elements included in its composition.
Alloyed alloys are distinguished. Special impurities are added to them to obtain the desired properties. From the point of view of the production process, alloys can be cast, powdered (sintered) and wrought.
The degree of connection of elements in an alloy can be different, so a distinction is made between a mechanical mixture (each element forms a separate crystal), a solid solution (different elements are built into a common crystal lattice) and a compound (atoms form a chemical bond).
To make iron more hard, carbon is added, but at the same time the metal becomes more brittle. Steel contains 0.3-2.14% carbon. Low-carbon steel is used as a structural material, while harder grades are used to make tools. Alloy steel is used in mechanical engineering and the manufacture of tools with high cutting speeds. Steel is alloyed by adding chromium, manganese, titanium, vanadium, etc. In this way, strength is increased without loss of hardness.
Cast iron contains from 2 to 4% carbon. Products with good abrasion resistance, strength, and rigidity are made from it by casting.
Cadmium slows down the wear of copper alloys. In copper alloys, zinc increases ductility and corrosion resistance. Titanium greatly increases the operating temperature limit. Nickel and, to a lesser extent, chromium increase the strength of ferrite without affecting ductility.
9th grade in chemistry
Where did the Pound Sterling come from?
Sterling is the name of an alloy of 92.5% (or higher) silver and 7.5% other metals, usually copper (sterling silver and higher). Pure .999 silver is too soft and unsuitable for creating large objects, so it is usually alloyed with copper to provide strength while maintaining the ductility and beauty of the precious metal.
The name "sterling" appeared in the 12th century. This was originally the name given to an ancient English silver coin. 240 coins weighed 1 pound (453.6 g). The cost of large purchases was calculated in pounds sterling. On the other hand, it was a way of checking the weight of the coins: if the weight of 240 coins was not equal to 1 pound, the coins could be counterfeit or too worn.
Share link
Popular message topics
- Shevardinsky redoubt.
Monument on the Borodino Field The Battle of Borodino is one of the heroic pages of Russian history. “It’s not for nothing that all of Russia remembers Borodin’s Day!” These lines by M.Yu. Everyone knows Lermontov by heart since childhood. The memory in the hearts of people about the bloodshed will not dry out - Human skin
The human skin performs many functions. The skin protects the human body from the external environment, harmful bacteria and microbes. Scientists have found that the skin is an organ, and the largest and heaviest, - Animals of the Red Book
For a long time, man has tried to live in an idyll with the animal world. Nature completely helped man to survive and adapt to the world. But over time, people stopped appreciating it. They began to pollute water bodies, destroy fertile
General characteristics of metal alloys
Now we will look at the general properties of metals and alloys by which they are characterized. They can very often be found in specialized literature.
| Characteristic | Decoding |
| Strength | The ability of an alloy to withstand mechanical loads and resist destruction. |
| Hardness | A property that determines the resistance of a material to attempts to introduce a part from another alloy or metal into its thickness. |
| Elasticity | The ability to restore its original shape after the application of significant mechanical force or load. |
| Plastic | On the contrary, this is a property that characterizes the possibility of changing shape and size under the influence of applied force, mechanical load. In addition, this also characterizes the ability of a part to maintain its newly acquired shape for a long time. |
| Viscosity | - the ability of a metal to resist rapidly increasing (shock) loads |
These are the qualities that characterize metal alloys. The table will help you understand them.
Production information
In principle, at present, an “alloy” may well be understood as a material based on only one chemical element, but “diluted” with a whole package of additives.
The most common method of obtaining them, melting them to a liquid state, has changed little since ancient times. For example, analysis of metals and alloys shows that the ancient Indians mastered a level of metal processing that was amazing for their time. They even began to create alloys using refractory zinc, which in our time is a rather labor-intensive and complex procedure.
Today, powder metallurgy is also widely used for these purposes. Ferrous metals and alloys based on them are especially often processed by this method, since in this case the maximum cheapness of both the process itself and the manufactured product is often required.
Examples of problem solving
| Exercise | How many kg of tin must be added to a piece of bronze (m=4kg) containing 15% tin in order to increase the tin content in it to 60% of the total mass? |
| Solution | Let two alloys be mixed, the second alloy containing 100% tin and no other components. Let's find the masses of tin in the alloys: |
Then the mass of alloys will be:
The ratio of the mass of tin in the new alloy to the mass of the new alloy is:
Source
Alloy classification
There are several ways to classify alloys:
- by manufacturing method (cast and powder alloys);
- by the method of obtaining the product (casting, wrought and powder alloys);
- by composition (homogeneous and heterogeneous alloys);
- according to the nature of the metal - base (ferrous - Fe base, non-ferrous - base, non-ferrous metals and alloys of rare metals - radioactive elements base);
- by the number of components (double, triple, etc.);
- by characteristic properties (refractory, low-melting, high-strength, heat-resistant, hard, anti-friction, corrosion-resistant, etc.);
- by purpose (structural, instrumental and special).
Read the chemistry essay: “Metal alloys” Page 1
(Back)
The “read” function is used to familiarize yourself with the work. The markup, tables and pictures of the document may be displayed incorrectly or not in full!
The metal objects around us rarely consist of pure metals. Only aluminum pans or copper wire have a purity of about 99.9%. In most other cases, people deal with alloys. Thus, various types of iron and steel contain, along with metal additives, small amounts of carbon, which have a decisive influence on the mechanical and thermal behavior of the alloys. All alloys have special markings, because... alloys with the same name (for example, brass) may have different mass fractions of other metals.
Various metals are used to make alloys. The most important among all alloys are steels of various compositions. Simple structural steels consist of relatively high purity iron with small (0.07-0.5%) additions of carbon. Thus, cast iron produced in a blast furnace contains about 10% other metals, of which approximately 3% is carbon, and the rest are silicon, manganese, sulfur and phosphorus. And alloy steels are obtained by adding silicon, copper, manganese, nickel, chromium, tungsten, vanadium and molybdenum to iron.
Nickel, along with chromium, is an essential component of many alloys. It gives steels high chemical resistance and mechanical strength. Thus, known stainless steel contains on average 18% chromium and 8% nickel. For the production of chemical equipment, aircraft nozzles, space rockets and satellites, alloys are required that are stable at temperatures above 1000 ° C, that is, they are not destroyed by oxygen and flammable gases and at the same time have the strength of the best steels. Alloys with a high nickel content satisfy these conditions. A large group consists of copper-nickel alloys.
An alloy of copper, known since ancient times, bronze contains 4-30% tin (usually 8-10%). Bronze products from masters of Ancient Egypt, Greece, and China have survived to this day. In the Middle Ages, tools and many other products were cast from bronze. The famous Tsar Cannon and Tsar Bell in the Moscow Kremlin are also cast from an alloy of copper and tin. Currently, in bronzes, tin is often replaced with other metals, which leads to a change in their properties. Aluminum bronzes, which contain 5-10% aluminum, have increased strength. Copper coins are minted from such bronze. Very strong, hard and resilient beryllium bronzes contain approximately 2% beryllium. Springs made of beryllium bronze are practically eternal. Bronzes made from other metals: lead, manganese, antimony, iron and silicon are widely used in the national economy.
Cupronickel alloy contains from 18 to 33% nickel (the rest is copper). The melting point of cupronickel is 1170 °C. It has a beautiful appearance. Cupronickel is used to make dishes and jewelry, and mint coins (“silver”). An alloy similar to cupronickel - nickel silver - contains, in addition to 15% nickel, up to 20% zinc. This alloy is used for the manufacture of artistic products and medical instruments. The copper-nickel alloys constantan (40% nickel) and manganin (an alloy of copper, nickel and manganese) have very high electrical resistance. They are used in the production of electrical measuring instruments. A characteristic feature of all copper-nickel alloys is their high resistance to corrosion processes - they are almost not subject to destruction even in sea water. Copper alloys
Properties of alloys
The properties of alloys depend on their structure. Alloys are characterized by structure-insensitive (determined by the nature and concentration of the elements that make up the alloys) and structure-sensitive properties (depending on the characteristics of the base). The structurally insensitive properties of alloys include density, melting point, and heat of evaporation. thermal and elastic properties, coefficient of thermal expansion.
All alloys exhibit properties characteristic of metals: metallic luster, electrical and thermal conductivity, ductility, etc.
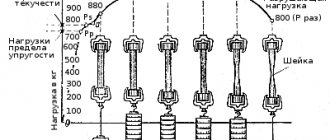
Also, all the properties characteristic of alloys can be divided into chemical (the relationship of alloys to the effects of active media - water, air, acids, etc.) and mechanical (the relationship of alloys to the effects of external forces). If the chemical properties of alloys are determined by placing the alloy in an aggressive environment, then special tests are used to determine the mechanical properties. So, to determine strength, hardness, elasticity, ductility and other mechanical properties, tensile, creep, impact strength, etc. tests are carried out.
About light alloys
As we have already said, the properties of metals and alloys differ in that the latter in many cases have higher characteristics. This is especially noticeable in relation to modern industry. In recent years, it has required a huge amount of light alloys, which have increased mechanical strength, as well as resistance to adverse environmental factors and high temperatures.
Most often, aluminum, beryllium, and magnesium are used for their production. Compounds based on aluminum and magnesium are especially in demand, since the scope of their possible application is extremely wide.