Not to be confused with Butene, Butyne, or Butane.
organic compound
butane
Butane[3]IUPAC systematic name
Tetracarban (never recommended[3])Other names
Butyl hydride;[1] Quartan;[2] Refrigerant 3-11-0 Identifiers CAS Quantity
- 106-97-8 Y
- Interactive image
- CHEBI: 37808 Y
- ChEMBL134702Y
- 7555Y
- 203-448-7
- D03186 Y
- 7843
- EJ4200000
- 6LV4FOR43R Y
- DTXSID7024665
- InChI = 1S / C4H10 / c1-3-4-2 / h3-4H2,1-2H3 Y
Key: IJDNQMDRQITEOD-UHFFFAOYSA-N Y
- SSSS
HOUR)11 nmol Pa−1 kg−1Conjugated acidButane Magnetic susceptibility (χ)-57.4·10−6 cm3/molThermochemistry Heat capacity ( C
)98.49 J K−1 mol−1 Std. Enthalpy of formation (Δl H
⦵298)−126.3–−124.9 kJ mol−1 Std. Enthalpy of combustion (Δc HOUR
⦵298)−2.8781–−2.8769 MJ mol−1Hazards[5]Safety Data Sheet See: Data Page GHS PictogramsGHS Signal Word Hazard Hazard Statements GHSH220 Precautionary Statements GHSP210 NFPA 704
(fire diamond) 4
1
0
SA Flash point -60 °C (-76 °F, 213 K) Autoignition temperature 405 °C (761 °F, 678 K) Explosion Limits 1.8–8.4% NIOSH
(US Health Exposure Limits): PEL (Permissible) none[ 1] REL (Recommended) TWA 800 ppm (1900 mg/m3)[1] IDLH (Immediate Hazard) 1600 ppm[1] Related Compounds Related Alkanes
- Propane
- Isobutane
- Pentane
), Dielectric constant (εр), etc. Thermodynamic dataPhase behavior solid–liquid–gas Spectral data, , NMR, Unless otherwise stated, data for materials are normalized to their standard state (at 25 °C [77 °F], 100 kPa).Y check (what is YN?) Links to infoboxes
Butane
(/ˈbjuːteɪp/) or
p
-butane
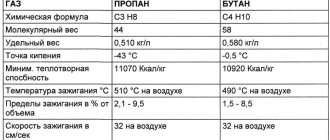
is an alkane with the formula C4H10. Butane is a gas at room temperature and atmospheric pressure. Butane is a highly flammable, colorless, easily liquefied gas that evaporates quickly at room temperature. The name butane comes from the roots no- (from Butyric acid, named after the Greek word for oil) and -an. This was discovered by chemist Edward Frankland in 1849.[6] It was discovered dissolved in crude oil in 1864. Edmund Ronalds, who first described its properties.[7][8]
Isomers
Main article: C4H10
| Common name | normal butane straight butane p -butane | isobutane i -butane |
| IUPAC name | butane | 2-methylpropane |
| Molecular diagram | ||
| Skeletal diagram |
Rotation about the central C−C bond produces two different conformations ( trans
and
tactless
) for
p
-butane.[9]
Reactions
The spectrum of a blue flame from a butane torch showing CH molecular radical emission bands and C2 Swan groups
When there is a lot of oxygen, butane burns to produce carbon dioxide and water vapor; when oxygen is limited, carbon (soot) or carbon monoxide. Butane is denser than air.
When there is enough oxygen:
2 C4H10 + 13 O2 → 8 CO2 + 10 h2O
When oxygen is limited:
2 C4H10 + 9 O2 → 8 CO + 10 h2O
The maximum adiabatic flame temperature of butane from air is 2,243 K (1,970 °C; 3,578 °F).
P
-Butane is a raw material for DuPont catalytic process for the preparation of maleic anhydride:
2 channels 3CH2CH2CH3 + 7 O2 → 2 C2HAS2(CO)2O + 8 H2O
p
-Butane, like all hydrocarbons, undergoes free radical chlorination, producing both 1-chloro- and 2-chlorobutanes, as well as more highly chlorinated derivatives. The relative chlorination rates are partly explained by different bond dissociation energies, 425 and 411 kJ/mol for the two types of CH bonds.
An excerpt characterizing Butane (substance)
– Yakov Alpatych got all worked up: another barrel was brought. - So listen. I’ll go to the police officer, and you tell the people, so that they give up this, and so that there are carts. “I’m listening,” answered Dron. Yakov Alpatych did not insist any more. He had ruled the people for a long time and knew that the main way to get people to obey was to not show them any doubt that they might disobey. Having obtained from Dron the obedient “I listen with,” Yakov Alpatych was satisfied with this, although he not only doubted, but was almost sure that the carts would not be delivered without the help of a military team. And indeed, by evening the carts were not assembled. In the village at the tavern there was again a meeting, and at the meeting it was necessary to drive the horses into the forest and not give out the carts. Without saying anything about this to the princess, Alpatych ordered his own luggage to be packed from those who had come from Bald Mountains and to prepare these horses for the princess’s carriages, and he himself went to the authorities. X After her father’s funeral, Princess Marya locked herself in her room and did not let anyone in. A girl came to the door to say that Alpatych had come to ask for orders to leave. (This was even before Alpatych’s conversation with Dron.) Princess Marya rose from the sofa on which she was lying and said through the closed door that she would never go anywhere and asked to be left alone. The windows of the room in which Princess Marya lay were facing west. She lay on the sofa facing the wall and, fingering the buttons on the leather pillow, saw only this pillow, and her vague thoughts were focused on one thing: she was thinking about the irreversibility of death and about that spiritual abomination of hers, which she had not known until now and which showed up during her father’s illness. She wanted, but did not dare to pray, did not dare, in the state of mind in which she was, to turn to God. She lay in this position for a long time. The sun set on the other side of the house and slanting evening rays through the open windows illuminated the room and part of the morocco pillow that Princess Marya was looking at. Her train of thought suddenly stopped. She unconsciously stood up, straightened her hair, stood up and went to the window, involuntarily inhaling the coolness of a clear but windy evening. “Yes, now it’s convenient for you to admire in the evening! He’s already gone, and no one will bother you,” she said to herself, and, sinking into a chair, she fell head first on the windowsill. Someone called her in a gentle and quiet voice from the side of the garden and kissed her on the head. She looked back. It was M lle Bourienne, in a black dress and pleres. She quietly approached Princess Marya, kissed her with a sigh and immediately began to cry. Princess Marya looked back at her. All previous clashes with her, jealousy towards her, were remembered by Princess Marya; I also remembered how he had recently changed towards m lle Bourienne, could not see her, and, therefore, how unfair were the reproaches that Princess Marya made to her in her soul. “And should I, who wanted his death, condemn anyone? - she thought. Princess Marya vividly imagined the position of m lle Bourienne, who had recently been distant from her society, but at the same time dependent on her and living in someone else’s house. And she felt sorry for her. She looked at her meekly questioningly and extended her hand. M lle Bourienne immediately began to cry, began to kiss her hand and talk about the grief that befell the princess, making herself a participant in this grief. She said that the only consolation in her grief was that the princess allowed her to share it with her. She said that all former misunderstandings should be destroyed before great grief, that she felt pure in front of everyone and that from there he could see her love and gratitude. The princess listened to her, not understanding her words, but occasionally looking at her and listening to the sounds of her voice. “Your situation is doubly terrible, dear princess,” said m lle Bourienne, after a pause. – I understand that you could not and cannot think about yourself; but I am obliged to do this with my love for you... Was Alpatych with you? Did he talk to you about leaving? – she asked. Princess Marya did not answer. She did not understand where and who was supposed to go. “Was it possible to do anything now, to think about anything? Doesn't it matter? She didn't answer. “Do you know, chere Marie,” said m lle Bourienne, “do you know that we are in danger, that we are surrounded by the French; It's dangerous to travel now. If we go, we will almost certainly be captured, and God knows... Princess Marya looked at her friend, not understanding what she was saying. “Oh, if only someone knew how much I don’t care now,” she said. - Of course, I would never want to leave him... Alpatych told me something about leaving... Talk to him, I can’t do anything and don’t want anything... - I talked to him. He hopes that we will have time to leave tomorrow; but I think that now it would be better to stay here,” said m lle Bourienne. - Because, you see, chere Marie, falling into the hands of soldiers or rioting men on the road would be terrible. - M lle Bourienne took out from her reticule an announcement on a non-Russian extraordinary paper from the French General Rameau that residents should not leave their homes, that they would be given due protection by the French authorities, and handed it to the princess. “I think it is better to contact this general,” said M lle Bourienne, “and I am sure that you will be shown due respect.” Princess Marya read the paper, and dry sobs shook her face. -Who did you get this through? - she said. “They probably found out that I’m French by name,” said m lle Bourienne, blushing. Princess Marya, with a paper in her hand, stood up from the window and, with a pale face, left the room and went to the former office of Prince Andrei.
Uses
Ordinary butane can be used for gasoline blending, as a solvent fuel gas for extracting flavors, alone or mixed with propane, and as a feedstock for the production of ethylene and butadiene, a key ingredient in synthetic rubber. Isobutane is primarily used by oil refineries to enhance (increase) the octane content of motor gasoline.[10][11][12][13]
When mixed with propane and other hydrocarbons, it may be called commercially LPG, for liquefied petroleum gas. Used as a component of gasoline, as a raw material for the production of base petrochemicals in steam cracking, as a fuel for cigarette lighters, and as a propellant in aerosols such as deodorants.[14]
Very pure forms of butane, especially isobutane, can be used as refrigerants and have largely replaced the ozone-depleting halomethanes in, for example, household refrigerators and freezers. System operating pressure for butane is lower than for halomethanes such as R-12. Thus, systems with R-12, such as automotive air conditioning systems, when converted to pure butane will not perform optimally, and therefore a mixture of isobutane and propane is used to provide cooling system performance comparable to R-12.
Butane is also used as a lighter fuel for a regular lighter or butane torch and is sold in bottles as a fuel for cooking, barbecues and camp stoves. The global butane canister market is dominated by South Korean manufacturers.[15]
As a fuel, it is often mixed with small amounts of hydrogen sulfide and mercaptans, which will give the unburned gas an unpleasant odor that is easily detected by the human nose. This way you can easily detect a butane leak. Although hydrogen sulfide and mercaptans are toxic, they are present in such low quantities that asphyxiation and fire hazards from butane become a problem long before toxicity.[ citation needed
] Most commercially available butane also contains a certain amount of contaminant oil, which can be removed by filtration, but which will otherwise leave a deposit at the flash point and can ultimately block the smooth flow of gas.[16]Contaminants are not used in flavor extraction.[
[clarify
] and butane gases can cause gas explosions in poorly ventilated areas if leaks go undetected and are ignited by a spark or flame.[
citation needed
]
| Canisters of butane fuel for use in camp stoves. | Butane lighter with liquid butane tank | An aerosol can that can use butane as a propellant. | Butane cylinder used for cooking |
Notes
- In turn, ancient Greek. βούτῡρον "butter" comes from βοῦς "cow, ox" and τυρός "cheese".
- [www.nge.ru/g_20448-90.htm GOST 20448-90. Hydrocarbon liquefied fuel gases for municipal consumption]
- [www.bestpravo.com/fed2003/data07/tex22892.htm Gas chromatographic measurement of mass concentrations of hydrocarbons: methane, ethane, ethylene, propane, propylene, n
-butane, alpha-butylene, isopentane in the air of the working area. Methodical instructions. MUK 4.1.1306-03 (APPROVED BY THE CHIEF STATE SANITARY DOCTOR OF THE RF 03/30/2003)] - Chemical Encyclopedia T1, M. 1988, p. 331, Article “Butanes”
- [fas.su/index.php?page=319 Physico-chemical properties of propane-butane mixture]
- [www.chemport.ru/chemical_substance_644.html) Butane: chemical and physical properties]
Consequences and health problems
Inhalation of butane can cause: euphoria, drowsiness, unconsciousness, asphyxia, cardiac arrhythmia, blood pressure fluctuations and temporary memory loss if abused directly from a high pressure container and can lead to death from asphyxiation and ventricular fibrillation. It enters the bloodstream and causes intoxication within seconds.[17] Butane is the most common volatile substance in the UK and was responsible for 52% of solvent-related deaths in 2000.[18] By spraying butane directly into the throat, the stream of liquid can quickly cool to -20 °C (-4 °F) by expansion, causing prolonged laryngospasm.[19] “Sudden death sniffer syndrome, first described by Bass in 1970,[20] is the most common cause of solvent-related death, causing 55% of known deaths.[19]
Physical properties
Butane is a colorless flammable gas with a specific odor, easily liquefies at normal pressure from −0.5 °C, freezes at −138 °C; at elevated pressure and normal temperature it is a highly volatile liquid. Critical temperature +152 °C, critical pressure 3.797 MPa.
- Solubility in water - 6.1 mg in 100 ml (for n
-butane, at 20 °C), much better soluble in organic solvents [4]). It can form an azeotropic mixture with water at a temperature of about 100 °C and a pressure of 10 atm. - Density of the liquid phase - 580 kg/m³[5]
- The density of the gas phase under normal conditions is 2.703 kg/m³, at 15 °C - 2.550 kg/m³ K: Wikipedia: Articles without sources (type: not specified)[ source not specified 2807 days
] - Heat of combustion 45.8 MJ/kg (2657 MJ/mol (see [6]).
Recommendations
- ^ a b c d e
NIOSH Pocket Guide to Chemical Hazards. "#0068". National Institute of Occupational Safety and Health (NIOSH). - Hofmann, August Wilhelm Von (1 January 1867). "I. On the effect of phosphorus trichloride on aromatic monamine salts.” Proceedings of the Royal Society of London
.
15
: 54–62. doi:10.1098/rspl.1866.0018. S2CID 98496840. - ^ a b
"Front matter."
Organic Chemistry Nomenclature: IUPAC Recommendations and Preferred Names 2013 (Blue Book)
. Cambridge: Royal Society of Chemistry. 2014. p. 4. Doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4. Likewise, the retained names "ethane", "propane" and "butane" were never replaced by the systematic names "dicarban", "tricarban" and "tetracarban", as recommended for the silane analogues, "disilane"; phosphane, "triphosphane"; and sulfan, "tetrasulfan". - W. B. Kay (1940). "Pressure-volume-temperature relationship for n-butane". Industrial and engineering chemistry
.
32
(3): 358–360. Doi:10.1021/ie50363a016. - “Safety Data Sheet, Material Name: n-Butane” (PDF). USA: Matheson Tri-Gas Incorporated. February 5, 2011. Archived from the original (PDF) October 1, 2011. Retrieved December 11, 2011.
- "Ok Paper" (PDF). www.chem.qmul.ac.uk.
_ - Watts, H. (1868). Dictionary of Chemistry
.
4
. item 385. - Maybery, C. F. (1896). "On the composition of sulfur oils of Ohio and Canada." Proceedings of the American Academy of Arts and Sciences
.
31
: 1–66. doi:10.2307/20020618. JSTOR 20020618. - Roman Mikhailovich Balabin (2009). "Enthalpy difference between conformations of normal alkanes: a Raman spectroscopy study of p
-Pentane and
p
-Butane".
J. Phys.
Chem. A .
113
(6):1012–9. doi:10.1021/jp809639s. PMID 19152252. - MarkWest Energy Partners, LP Form 10-K. Sec.gov
- Copano Energy, LLC Form 10-K. Sec.gov. Retrieved December 12, 2012.
- Targa Resources Partners LP Form10-k. Sec.gov. Retrieved December 12, 2012.
- Crosstex Energy, LP FORM 10-K. Sec.gov
- Primer for mixing gasoline. EPRINC Information Memorandum
- “The entrepreneur overcame the hardships of a Chinese prison.” houstonchronicle.com
. June 21, 2016. Retrieved September 20, 2022. - "BHO Mystery Oil". Skunk Farm Research
. 2013-08-26. Received 2019-12-05. - "Neurotoxic effects from butane gas." thcfarmer.com. December 19, 2009. Retrieved October 3, 2016.
- Field-Smith M, Bland JM, Taylor JS, et al. “Trends in mortality associated with volatile substance abuse, 1971–2004.” (PDF). Department of Public Health Sciences. London: St George's Medical School. Archived from the original (PDF) on March 27, 2007.
- ^ a b
Ramsey, J., Anderson, H. R., Bloor, K., et al. (1989).
"Introduction to the practice, prevalence and chemical toxicology of volatile substance abuse". Hum Toxicol
.
8
(4): 261–269. Doi:10.1177/096032718900800403. PMID 2777265. S2CID 19617950. - Bass M. (1970). "Sudden snuff death." JAMA
.
212
(12):2075–2079. Doi:10.1001/jama.1970.03170250031004. PMID 5467774.
Links
| Alkenes | Ethylene • Propene • Butenes • Pentenes • Hexenes • Heptenes • Octene |
| Alkynes | Acetylene • Propyne • Butine |
| Dienes | Propadiene • Butadiene • Isoprene • Cyclobutadiene |
| Other unsaturated | Vinylacetylene • Diacetylene • Carotene |
| Cycloalkanes | Cyclopropane • Cyclobutane • Cyclopentane • Cyclohexane • Cyclooctane • Decalin • Indane • Indene |
| Aromatic | Benzene • Toluene • Dimethylbenzenes • Ethylbenzene • Propylbenzene • Cumene • Styrene • Phenylacetylene • Indane • Diphenyl • Diphenylmethane • Triphenylmethane • Tetraphenylmethane • Indene |
| Polycyclic | Naphthalene • Anthracene • Benzanthracene • Pentacene • Phenanthrene • Pyrene • Benzpyrene • Azulene • Chrysene |