Propane butane is the basis for creating LPG - liquefied petroleum gas. With a slight increase in pressure, the mixture changes its state of aggregation to liquid, which is economically beneficial from the point of view of transportation, storage and use, both for industrial and domestic needs.
Liquefied propane butane is isolated during the distillation of oil (associated oil and gases) or obtained from gas condensate fields. During the processing process, they are purified from ethane, and gas gasoline is also released.
Where is propane used?
Due to its high combustion temperature, propane is widely used as a fuel for cars, as a fuel element for gas stoves and heating systems. At enterprises it is used in the process of cutting metal and welding metal structures. In the food and chemical industries it is used as a solvent or food additive.
Propane-butane mixture
What is the difference between butane and propane
- Propane has the chemical formula C3H8, and butane has the chemical formula C4H10. These gases have similar molecular weights and thermal conductivities. The main difference between these alkanes and each other is their boiling point.
- Butane has a boiling point of about -1 degrees Celsius, while propane has a boiling point of -43 degrees Celsius. When these values are reached, butane and propane pass from a gaseous state to a liquid state, i.e. liquefy.
- This leads to different specifics of using these gases.
- Butane is less resistant to low temperatures, so it is difficult to use in the cold season.
- Propane has another significant drawback - it is dangerous to use as a fuel when exposed to high temperatures. When heated, propane expands and presses on the walls of the gas tank or cylinder. This can lead to cracks in the walls of the vessel and to its explosions.
Propane is more explosive because it cannot withstand prolonged exposure to high temperatures.
Why do they mix propane and butane?
At subzero temperatures, butane in its pure form loses its basic properties. And propane is not applicable in hot climates.
To avoid the need to produce a different type of gas for each region according to the temperature regime, GOST provides for a mixture of these two components in a certain ratio.
Technological factor
In addition to the climatic factor, there is a technological need for combining propane with butane. At oil refineries, these two gases are produced in different volumes. Therefore, within the framework of raw materials policy, it is beneficial to mix them together in a certain proportion.
LPG can easily be converted from a liquid to a gaseous state, and thanks to the combination of temperature conditions of propane and butane, their mixture as a whole is highly resistant to freezing and boiling.
Methods for producing propane-butane
In the process of processing petroleum gases and oil itself, ethane, propane, butane, and gas gasoline are extracted at refineries.
Pricing policy for LPG refueling
The cost of propane-butane depends on the content of the first (more expensive) component. Therefore, it is not surprising that the “winter” mixture for refueling an autonomous gas supply system will be more expensive than the “summer” one. However, if any company offers refueling at a price significantly lower than the market average, then its representative should ask the following questions:
- Why is the cost of LPG so low?
- What is the propane to butane ratio?
- How will this composition work in winter?
- Is appropriate technical documentation available?
- Can I contact the company if problems arise?
Be careful! A cheap mixture can then cost much more.
Some companies cheat by providing a “winter” mixture that does not comply with GOST. Therefore, the low cost of LPG should, at a minimum, alert the buyer.
For autonomous gasification of a home, the quality of gas is of decisive importance, since the reliability of the entire system depends on it. Therefore, it is important to understand why propane is mixed with butane, and in what proportion these components should be used in winter and summer. Read more about gas supply to private properties in the article: autonomous heating with propane-butane.
To avoid problems with gasification of your home, contact a company that has already proven its professionalism and reliability. This is evidenced by our good position in the market and the absence of negative feedback from customers.
Chemical and physical properties
Propane-butane is odorless. However, it is highly explosive. Therefore, in order to be able to immediately detect a leak if something happens, a strong-smelling odorant substance, ethyl mercaptan, must be added to this mixture.
Due to the property of the appearance of vapor pressure in the presence of a liquid phase in the cylinder, this gas is convenient to store in small volumes.
Dependence of saturated vapor pressure of propane-butane mixture on temperature
When used as a fuel mixture, butane is quite high in calories, and propane evaporates easily at low temperatures. Therefore, the ratio of these substances in the mixture depending on the temperature conditions plays an important role.
As a rule, the lower the temperature, the more propane should be in the mixture (70-80%). In summer, it is enough for the propane content in the mixture to be about 40%.
Main performance characteristics
The propane-butane mixture can have a different percentage composition. The most common are mixtures with the following gas ratio:
- Winter, propane: butane – 75% to 25%;
- Summer, propane: butane - 55% to 45%, respectively.
IMPORTANT! If the butane content in the mixture exceeds 60%, then in the climate of most of the European territory of Russia, the operation of tank units may be difficult. To ensure uninterrupted operation, the gas tank is equipped with additional equipment - an LPG evaporator.
Propane butane gas is characterized by the following operational characteristics and requirements for equipment in which mixtures are transported and stored:
- The high elasticity of evaporation of the liquid fraction is used for gravity delivery of gas from the gas tank to the consumer;
- The mixture is odorless, so odorization with various substances is performed to detect leaks. The most commonly used is ethylmer captan;
- Temperature range of flammability limit: for butane 430°C for propane – 504°C. The flammability limit is 1.9% and 2.3% respectively;
- A propane-butane mixture in any modification is heavier than air. If the integrity of the containers is compromised, it accumulates in the lower part of the premises, inspection wells, etc. Therefore, equipment operating on these mixtures is not installed in basements, boiler rooms, etc.;
- When 1 m3 of mixture is burned, its air requirement is determined depending on the composition at the rate of 24 m3 for propane and 31 m3 for butane. Therefore, technical rooms and boiler rooms where propane butane is used as fuel must have forced ventilation;
- The coefficient of volumetric expansion of the gas mixture is 16 times greater than that of water. Therefore, gas tanks and conventional cylinders are not filled to more than 85%. Exceeding this volume may lead to a violation of the integrity of the structure;
- When 1 kg of gas mixture evaporates, a gas with a volume of 450 liters is formed. in fact, to obtain 1 m3 of vapor phase, it is necessary to evaporate 2.2 liters. This volume is sufficient to obtain thermal energy of about 25.3 kWh.
Transportation and storage
Since evaporation of liquid in LPG occurs even at 0 °C, these gases are usually stored strictly in closed containers.
Large consumers receive hydrocarbon gases in railway or road tanks, from which they are transferred to factory stationary containers.
Small consumers usually use cylinders.
Transport gases in accordance with the rules for the transportation of dangerous goods and the rules for the safe operation of pressure vessels.
First of all, it is worth considering the properties of propane. Its temperature in the cylinder should not exceed 15 degrees Celsius, otherwise there is a risk of fire.
Physico-chemical properties of propane-butane mixture
The great advantage of propane-butane mixtures is their similarity in basic characteristics to traditional motor fuels. It is this quality that has allowed them to take a strong position in the market. The hydrocarbons that make up associated petroleum gas are in a gaseous state under normal conditions, but with an increase in external pressure they change their state of aggregation and turn into liquid. This property makes it possible to achieve high energy density and store liquefied hydrocarbon gas (LPG) in tanks that are relatively simple in design. Unlike associated petroleum gas, the hydrocarbons that make up natural gas are in a gaseous state under normal conditions and do not change their state of aggregation even with a significant change in pressure. Therefore, storing compressed natural gas (CNG) is associated with significant difficulties - for example, the tank must withstand significant pressure of up to 200 atmospheres. Technologies for the production and use of liquefied natural gas (LNG), which can be stored in special isothermal vessels at temperatures below -160°C and a pressure of about 40 bar, are being intensively promoted. In many ways, the benefits of the high energy density of LNG are lost due to the complexity of cryogenic equipment, which is much more expensive and requires constant monitoring by highly qualified personnel. LPG production The main components of liquefied hydrocarbon gas are propane C3H8 and butane C4H10. Mainly industrial production of liquefied gas is carried out from the following sources:
- associated petroleum gases;
- condensate fractions of natural gas;
- gases from oil and condensate stabilization processes;
- refinery gases obtained from oil refining plants.
Table 1. Physico-chemical parameters of liquefied hydrocarbon gas according to GOST 27578-87
| Index | Brand GSN | |
| PA | PBA | |
| Mass fraction of components, %: | ||
| methane and ethane | Not standardized | |
| propane | 90±10 | 50±10 |
| hydrocarbons C4 and higher | Not standardized | |
| unsaturated hydrocarbons, (no more) | 6 | 6 |
| Volume of liquid residue at +40°С, % | Absent | |
| Saturated vapor pressure, MPa: | ||
| at +45°C, no more | – | 1,6 |
| at -20°С, not less | – | 0,07 |
| at -35°С, not less | 0,07 | – |
| Mass fraction of sulfur and sulfur compounds, %, no more | 0,01 | 0,01 |
| Including hydrogen sulfide,%, no more | 0,003 | 0,003 |
| Free water and alkali content | Absent | |
The component composition of liquefied gas is regulated by technical standards GOST 27578-87 “Liquefied hydrocarbon gases for road transport. Technical conditions" and GOST 20448-90 "Hydrocarbon liquefied fuel gases for municipal consumption. Technical conditions". The first standard describes the composition of liquefied gas used in road transport. On the website of the Technosoyuz company, paint booths are presented in a wide range, as well as various equipment for car service. In winter, it is prescribed to use liquefied gas of the PA brand (propane for automobiles), containing 85 ± 10% propane, in the summer - PBA (propane-butane for automobiles), containing 50 ± 10% of propane, butane and no more than 6% of unsaturated hydrocarbons. GOST 20448-90 has wider tolerances for the content of components, including those harmful from the point of view of their impact on gas equipment (for example, sulfur and its compounds, unsaturated hydrocarbons, etc.). According to these technical conditions, gas fuel is supplied in two grades: a winter propane-butane mixture (SPBTZ) and a summer propane-butane mixture (SPBTL). The PBA gas grade is allowed for use in all climatic regions at ambient temperatures not lower than -20°C. The PA brand is used in winter in those climatic regions where the air temperature drops below -20°C (the recommended range is -25...-20°C). In the spring, in order to fully exhaust reserves of PA grade liquefied gas, its use is allowed at temperatures up to 10°C. Pressure in the cylinder In a closed tank, LPG forms a two-phase system. The pressure in the cylinder depends on the saturated vapor pressure (vapor pressure in a closed volume in the presence of a liquid phase) and characterizes the volatility of the liquefied gas, which, in turn, depends on the temperature of the liquid phase and the percentage of propane and butane in it. The volatility of propane is higher than that of butane, which is why its pressure at negative temperatures is higher. The experience of many years of practical operation shows:
- at low ambient temperatures, it is more effective to use LPG with a high propane content, since this ensures reliable evaporation of gas and, consequently, a stable supply of the product;
- at high positive ambient temperatures, it is more effective to use LPG with a reduced propane content, otherwise significant excess pressure will be created in the tank and pipelines, which can negatively affect the tightness of the gas system.
In addition to propane and butane, LPG contains a small amount of methane, ethane and other hydrocarbons, which can change the properties of the mixture. Thus, ethane has a higher saturated vapor pressure compared to propane, which can have a negative effect at positive temperatures. Change in the volume of the liquid phase when heated The propane-butane mixture has a large coefficient of volumetric expansion of the liquid phase, which for propane is 0.003, and for butane - 0.002 per 1°C increase in gas temperature. For comparison: the coefficient of volumetric expansion of propane is 15 times, and butane is 10 times greater than that of water. Technical standards and regulations establish that the degree of filling of tanks and cylinders depends on the type of gas and the difference in its temperature during filling and during subsequent storage. For tanks whose temperature difference does not exceed 40° C, the filling degree is assumed to be 85%; with a larger temperature difference, the filling degree should be reduced. Cylinders are filled by weight in accordance with the instructions of the “Rules for the design and safe operation of pressure vessels.” The maximum permissible heating temperature of the cylinder should not exceed 45°C, while the vapor pressure of butane reaches 0.385 MPa, and of propane - 1.4–1.5 MPa. Cylinders must be protected from heating by sunlight or other heat sources. Change in gas volume during evaporation When 1 liter of liquefied gas evaporates, about 250 liters of gaseous gas is formed. Thus, even a minor leak of LPG can be very dangerous, since the volume of gas during evaporation increases by 250 times. The density of the gas phase is 1.5–2.0 times greater than the density of air. This explains the fact that when there is a leak, the gas has difficulty dispersing into the air, especially indoors. Its vapors can accumulate in natural and artificial depressions, forming an explosive mixture. Table 2. Physico-chemical properties of the components of liquefied gas and gasoline.
| Index | Propane | Butane (normal) | Petrol |
| Molecular mass | 44,10 | 58,12 | 114,20 |
| Density of the liquid phase under normal conditions, kg/m3 | 510 | 580 | 720 |
| Gas phase density, kg/m3: | |||
| under normal conditions | 2,019 | 2,703 | – |
| at a temperature of 15°C | 1,900 | 2,550 | – |
| Specific heat of evaporation, kJ/kg | 484,5 | 395,0 | 397,5 |
| Lower combustion heat: | |||
| in liquid state, MJ/l | 65,6 | 26,4 | 62,7 |
| in gaseous state, MJ/kg | 45,9 | 45,4 | 48,7 |
| in gaseous state, MJ/m3 | 85,6 | 111,6 | 213,2 |
| Octane number | 120 | 93 | 72-98 |
| Flammability limits when mixed with air under normal conditions, % | 2,1–9,5 | 1,5–8,5 | 1,0–6,0 |
| Self-ignition temperature, °C | 466 | 405 | 255–370 |
| Theoretically required amount of air for combustion of 1 m3 of gas, m3 | 23,80 | 30,94 | 14,70 |
| Volumetric expansion coefficient of the liquid fraction, % per 1°C | 0,003 | 0,002 | – |
| Boiling point at a pressure of 1 bar, °C | -42,1 | -0,5 | +98…104 (50% point) |
Scope of gas application
Propane-butane is a unique gas-based substance that contains molecules of the same name.
The generally accepted chemical formula of propane consists of molecules and atoms of two main components - propane (C3H8) and butane (C4H10).
Widely used for domestic purposes, this gas is used almost everywhere - from cooking food in a frying pan, to cutting a thick layer of metal, to its active use in various industries in general.
It is also increasingly used by people who have given up gasoline-based fuel to fuel their cars.
Table
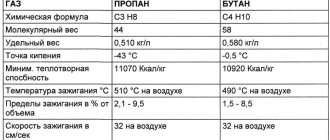
The answer to the question, what is the difference between propane and butane, is obvious. These compounds of carbon and hydrogen, standing next to each other in a homologous series, differ slightly from each other. The difference in their properties depends primarily on the different number of atoms of their constituent simple substances, which contain molecules of the compounds.
| Propane | Butane | |
| Chemical formula | С3Н8 | С4Н10 |
| What is | Colorless, odorless, flammable gas | Colorless, odorless, flammable gas |
| Physical properties | Melting point at normal atmospheric pressure –187.6 °C; boiling point –42.09 °C | Melting point at normal atmospheric pressure –138.4 °C; boiling point –0.5 °C |
| Usage | Raw materials for the chemical industry, widely used as fuel, food industry (food additive E944), can be used as a refrigerant in refrigeration units | Raw materials for the chemical industry, used as fuel (the range of application is narrower than that of propane), food industry (food additive E943), can be used as a refrigerant in refrigeration units |
How to store propane-butane?
You can learn about the storage features of the substance by following the rules prescribed in GOST. Propane butane must be kept under high pressure in specialized underground storage facilities, tanks or metal cylinders. It is imperative to secure this place so that there are no impacts on the container, overheating and other factors that could cause an explosion. This applies to both full and empty containers. Do not store propane-butane near other explosive substances, toxic and flammable materials, as well as oxidizers.
As you can see, the technical mixture is quite dangerous, but nevertheless very important for the normal functioning of many industries.
Technical propane-butane: technical characteristics
According to GOST, technical characteristics must normally correspond to the following indicators:
- the mass fraction of butylenes and butanes should not exceed 60%;
- the amount of liquid remaining at a temperature of 20⁰C should not exceed one and a half percent;
- pressure at a temperature of 45 ⁰C should be 16 atmospheres;
- the amount of hydrogen sulfide and mercaptanoic acid should not exceed 0.013% and 0.003%, respectively;
- the propane-butane composition should not contain alkali or water;
- odor intensity should not exceed 3 points.
In addition, propane-butane is explosive. The slightest flame, and even a spark, can cause a strong fire. When interacting with air, the mixture forms explosive substances.
Alkanes
Other names for this class of hydrocarbon compounds are paraffins, as well as saturated aliphatic hydrocarbons. These are acyclic compounds of carbon and hydrogen, in which the atoms form straight or branched chains; The general formula of alkanes is CnH2n+2. In this formula, C are carbon atoms, H are hydrogen atoms, and the subscripts indicate their number in the molecule. The first in the series of alkanes is methane, which has the formula CH4. Propane and butane with the formulas (respectively) C3H8 and C4H10 occupy the third and fourth positions.
The difference between propane and butane is not only in the number of carbon and hydrogen atoms in the molecule; the properties of the compounds are also different. The first thing that catches your eye when studying their physical properties is the different conditions for the transition from one state of aggregation to another (that is, from solid to liquid and then to gaseous) at normal atmospheric pressure. For example, propane goes from solid to liquid (that is, “melts”) at a temperature of –187.6 degrees Celsius, and from liquid to gas (“boils”) at a temperature of –42.09 degrees. For butane, these figures are different: it melts at a temperature of -138.4 degrees, boils at a temperature of -0.5 degrees. Both gases are poorly soluble in water.
The difference between propane and methane
Among the distinctive features of propane it is worth noting:
- higher combustion efficiency, making it much more efficient than methane during welding;
- high inertness of the gas, which allows it to more actively enter into various chemical reactions;
- propane is safer than methane and has a narcotic effect;
- When transporting propane, you do not need to use any special equipment; ordinary steel cylinders are sufficient.
In addition, propane is cheaper and easier to refill.