Features of Graphite Crucible
Graphite crucibles for melting non-ferrous metals have a fairly long service life.
They resist oxidation, thermal and mechanical effects of the melt well. Crucibles are mainly used in conjunction with induction heating furnaces. Graphite Crucible Induction Furnace
Graphite, as a material, has the following properties:
- heat resistance;
- fire resistance;
- high thermal conductivity;
- increasing strength characteristics when heated;
- low specific gravity;
- low porosity;
- prevention of oxidation;
- resistance to: corrosion;
- sticking;
- burnt.
Graphite crucibles are not made from pure graphite. To form the mixture, refractory clay (fireclay, alumina) and quartz sand are added to the graphite. Some of the clay can be replaced with kaolin. High-quality containers can easily carry a significant number of heats.
For steel melting, the composition of the charge mixture is selected according to the requirements for the purity and characteristics of the steel.
| Batch materials, % | Graphite | Alumina | Kaolin | Chamotte |
| Alloy type | ||||
| High strength steel | 54 | 35 | — | 10 |
| Medium hard steel | 40 | 38 | — | 22 |
| Razor steel | 12 | 40 | 40 | 8 |
| Quality steel (clean) | 3 | 87 | 10 | — |
| Copper | 8 | 67 | — | 25 |
| Brass, bronze | 12 | 50 | 13 | 25 |
| Gray cast iron | 53 | 43 | — | 4 |
| Modified cast iron | 50 | 40 | — | 10 |
For smelting copper and cast iron, Blaninger proposed the following proportions of crucible charges.
| Batch materials, % | Copper alloys | Cast iron | ||||
| A | B | C | D | E | F | |
| Graphite | 48 | 57,5 | 55 | 50 | 58 | 55 |
| Clay | 32 | 25,5 | 35 | 40 | 35 | 30 |
| Kaolin | 6 | 10,5 | 5 | — | — | 7 |
| Quartz sand | — | — | — | 5 | 7 | 8 |
| Chamotte | 14 | — | 5 | 5 | — | — |
| Silicon sand | — | 6,5 | — | — | — | — |
An important production indicator is the cost of finished products with given characteristics. Therefore, various types of graphite are used to make crucibles.
Grain graphite is not used. Lamellar or crushed into a small fraction is used. Large-lamellar graphite is fire-resistant, heat-resistant and has a high density. Fine-plate graphite (amorphous) is less resistant and the number of melts in it is much less. The strength characteristics of crucibles depend on the ash content of graphite.
Graphite crucibles for steels
Crucibles for steels are made of highly concentrated graphite, the content of which reaches 90%. But the presence of iron oxides should be kept to a minimum. When smelting copper, graphite is partially replaced by retort graphite or coke.
Recently, the following grades of graphite have found widespread use:
- EG2 – electrographite;
- GM – fine-grained graphite;
- MPG – isostatic graphite.
Electrographite
Electrographite EG2 differs from the graphite from which electrodes are made in that it has less porosity. This grade is recommended for re-melting or for melting with slag removal.
Fine-grained graphite
Fine-grained graphite GM is recommended for melting pure metals. Resistant to burning.
Isostatic graphite
Isostatic graphites PGMs have the best characteristics, and therefore high cost.
Characteristics of copper
Copper is one of the first metals that man learned to mine and process. Products made of copper and its alloys were used as early as the 3rd century BC, as evidenced by historical data and the results of archaeological excavations. The widespread use of copper has largely been facilitated by the fact that it can be quite easily processed by various mechanical methods. In addition, it can be easily melted.
Copper, the surface of which has a distinct yellowish-red color, due to its softness, can easily be processed by plastic deformation. When the surface of copper interacts with the surrounding air, it becomes covered with an oxide film, which gives it such a beautiful color.
Grades of technical copper and their chemical composition
The characteristics of copper, such as electrical and thermal conductivity, are also of great importance, for which it ranks second among all metals, second only to silver. Due to these properties, products made from it are actively used in the electrical industry, as well as in cases where it is necessary to ensure rapid heat removal from a heated object.
Another important parameter of copper, which directly affects the amount of energy and labor consumed in the production of products from it, is the melting point. For pure copper, the temperature at which the metal changes from solid to liquid is 1083°. If you mix copper with tin and get bronze, then the melting point of such an alloy will already be 930–1140°, depending on the content of the main alloying additive in it. A copper alloy such as brass, which is obtained by adding zinc to the base metal, has an even lower melting point, which is in the range of 900–1050°.
Electrical properties of copper at a temperature of 20°
If you decide to implement a technological process such as copper casting at home, it is important to know one more parameter - its boiling point. At 2560° copper begins to literally boil, which is clearly visible in the video of this process
The appearance of bubbles on the surface of the liquid metal and active gas formation in it is facilitated by carbon released from copper as a result of its oxidation, which occurs during strong heating.
If the smelting technology is followed, shallow pores may remain on the surface of the copper ingot, which can be easily removed by grinding
Heating methods
If you need to melt more than 150-200 g of metal at a time, then you will need to build a crucible furnace next to the crucible, otherwise it will be very difficult to achieve homogeneity of the melt and high quality casting. The exception is low-melting and easily recoverable lead: up to 20-30 kg of it can be melted at a time at home. A relative exception is zinc for hot galvanizing; its melt in a crucible without a furnace can be up to 2-2.5 kg, but borax must be sprinkled on top of it so that the surface of the melt is completely covered with its fluidized layer. Steel fasteners are thrown into the melt through a layer of borax.
The optimal method in all respects for heating the crucible in a furnace is with gas, pos. 1 in Fig., but a gas crucible furnace is a rather complex structure, although it can easily be made independently. The most suitable crucible for a gas furnace is a graphite ceramic crucible, because its material has fairly high thermal conductivity. If there are particularly high requirements for metal purity, it is better to use a neutral ceramic crucible. When lower for fusible metals - cast iron, as it conducts heat better and thereby saves fuel. Graphite crucibles are placed in a gas furnace only if strong reduction of old oxidized metal is required, and the danger of carburization is insignificant, for example, when melting silver extracted from the earth for refining
Methods for melting metal in a crucible
For low-melting metals, the electric crucible furnace, pos. 2; it may be the so-called ohmic (with heating by a nichrome spiral) or induction, with heating from an electromagnetic oscillation generator, see below. Only ceramic neutral or, to a limited extent, graphite crucibles are suitable for induction furnaces.
If the crucible contains more than 2-2.5 kg of metal, then according to safety rules the crucible furnace must be made tiltable (item 3), because and 1 kg of melt spilled on the floor is already a big disaster. On the contrary, it is preferable to heat metal in small jewelry crucibles without a furnace, directly with the flame of a burner, pos. 4. In this case, the crucible is held throughout the melting process with a special spring grip, pos. 5 and 6.
Note: silver and its alloys, as well as lead on sinkers, can be melted at home in quantities of up to 15-20 g, using instead of a crucible... a food-grade stainless steel spoon, see fig. on right. For safety, then it is necessary to make gaskets for the jaws of the vice with longitudinal cuts under the handle of the spoon. The flame is exclusively gas; gasoline can burn a spoon.
Read also: Silver electroplating at home
Electric heating
Ohmic crucible furnaces are mainly used for smelting lead or tin. For more refractory metals, they turn out to be uneconomical, but up to 20 kg of lead can be melted at a time in a home crucible electric furnace; how to make your own electric crucible for melting lead, see for example. video:
Video: electric crucible for melting lead
Melting aluminum in a crucible turns out to be more profitable by induction due to its high electrical conductivity, but this trick no longer works with copper - its temperature and latent heat of fusion are much higher. With the induction melting method, the metal is heated by Foucault eddy currents, for which the crucible with it is placed in an EMF coil of thick copper wire, powered by alternating current from an electromagnetic oscillation generator. How to make a generator with your own hands for inductively heating small amounts of metal, for example, for trinkets, is described in other materials, or, for example, see next. video guide.
Video: DIY induction heating
Induction crucible furnace for aluminum melting
With an increase in the amount of melted metal, not only does the required power of the generator increase, but its optimal frequency also decreases, this affects the so-called. surface effect (skin effect) in metal. If 100-200 g of aluminum can be melted into EMF from any homemade generator for inductive heating, then installation of 1.5-2 kg of duralumin or magnesium alloy is already a solid structure, see fig. on right. If you intend to work with aluminum, then think carefully - is it worth building something like this? Wouldn't it be easier to use a mini gas furnace for melting small quantities of aluminum alloys, see for example. video clip
A little about melting
In a deep vacuum, the high-purity metal being melted can be heated exactly to the melting temperature or slightly higher, and kept at it for some time so that tiny, literally a few atoms, remains of crystallites melt. Then the metal can be allowed to cool slightly below its melting point - it will remain liquid, like a supersaturated solution without a seed crystal. If we now pour the metal, also in a vacuum, into a mold made of a chemically absolutely inert material, in which a seed crystal of the same metal is placed, then, observing all the subtleties of this technology, we will obtain a single-crystal casting with unique properties.
In amateur conditions, vacuum melting, alas, is not feasible. In order to properly make a crucible for melting metal yourself, you need to take into account a number of features of melting in a non-inert chemical gas environment. The melted metal, firstly, interacts with air, causing part of it to be lost to the formation of oxide, which is especially important when melting scrap precious metals: at its melting temperature (1060 degrees Celsius), even gold noticeably oxidizes. To compensate to some extent for oxidation, the crucible must provide a reducing environment for the melt or be chemically inert if the metal is melted with a clean open flame, see below.
Secondly, so that the metal in the crucible does not freeze until it is brought to the casting mold, so that the remnants of the original crystallites do not spoil the casting, and the melt acquires sufficient fluidity, the metal in the crucible is overheated. For example, the melting point of zinc is 440 degrees, and its foundry temperature is 600. Aluminum, respectively, 660 and 800. Since overheating of the metal after melting takes some time, degassing of the melt also occurs at the same time, this is the third thing.
Recovery
In metallurgy, atomic carbon C, carbon monoxide CO (carbon monoxide) and hydrogen H are used as reducing agents. The latter is most often an accidental guest, because for this purpose it is too active and is absorbed by metals without forming chemical compounds with them in large quantities, which spoils the casting material. For example, solid platinum at room temperature can absorb up to 800 volumes of hydrogen. A platinum blank in a hydrogen atmosphere literally swells before our eyes, cracks and falls into pieces. If you take them out of the hydrogen chamber and heat them, hydrogen will be released back.
Note: in a similar way, but in smaller quantities, metals absorb/emit other gases, e.g. nitrogen. This is why degassing of the melt is required, see also below.
A noticeable proportion of hydrogen reduction occurs when heated by an open flame of a gas burner, upon its contact with a less heated surface. The metal does not deteriorate - the absorbed hydrogen is released and burned later in the smelting process. But, if the crucible material is also prone to gas absorption, it may crack and burst during melting; this must be kept in mind.
CO reduction is noticeable if the metal in the crucible is melted by the open flame of a liquid (gasoline, kerosene, diesel) burner, for the same reasons. Liquid fuel burns much slower than gas, and its afterburning zone extends several cm from the burner nozzle. Reduction with carbon monoxide is the cleanest from the point of view of the metal: it does not spoil the metal and does not produce by-products with a strong excess of the reducing agent. Therefore, CO reduction is widely used in metallurgy when smelting metal from ore, but no one has yet figured out how to make a crucible furnace (see below), in which oxidation compensation would be completely provided by CO.
Making a clay crucible
Here you can’t do without fireclay clay, which is sold in any building materials store. It tolerates extreme thermal effects very well, is cheap and there are unlikely to be any problems finding it. As a last resort, you can make a crucible from crushed fireclay bricks. You will also have to buy liquid glass and mix all the ingredients for a homogeneous base. The proportions look something like this:
- 7 units of clay;
- 3 units of fireclay;
- 10 spoons of liquid glass.
Clay crucibles
All components are added in stages: clay and fireclay are mixed until smooth, and water is gradually added to them. The main goal is to create a mixture that will not stick to your hands. When the required consistency is obtained, glass is added and everything is thoroughly mixed. The main thing here is to bring the object to a state where the plane stops cracking. The mixture is ready, and for storage it is recommended to use thick cellophane, or wrap it in 7-10 layers of film.
The mixed material is applied inside the model, its depth and thickness are formed. It is better to create a semicircular bottom, which will give a greater effect during future melting of iron filings. Also, the substance must be pressed tightly against the model so that air does not form between the planes, and for greater convenience it is recommended to wet your hands with water.
Afterwards, the tank is sent for drying: it is placed in a container made of cardboard or plastic and placed in a dry place. A few hours will be enough to remove any remaining moisture. Also, the product will settle a little, and it will be easy to remove it from the mold. A refractory vessel made of fireclay bricks will be enough for a long time of use, however, the last point of creation should be the firing procedure in a furnace and at T = 800 °C. And the thing can be used for its intended purpose. For ease of use, you will need a crucible furnace, which you can make yourself. For simple installation, you can weld a structure from several pipes to form a cylinder. Usually it is fixed on two parallel posts so that it does not touch the ground. And here the thickness of the walls (minimum 5 mm) and the stability of the product are taken into account (it must easily withstand T = 1600 °C or more).
How to make a graphite crucible
Capacity of this category has many advantages:
- low overall weight;
- resistance to hot alloys;
- good thermal conductivity;
- strength increases with increasing temperature.
If you take the easy route, you can take a graphite rod, and the crucible is almost ready. All that remains is to attach the bottom.
Graphite crucibles in various sizes
If the required tube is not found, everything can be done using two molds of different sizes, which are inserted one into the other, and the free space will allow you to give the desired dimensions. Initially, you need to pour the mortar into an empty container, and you should not spare it. The fact is that the powder will compact and settle. Next, add liquid glass (about 15 ml) and mix everything thoroughly. It is recommended to place the mixed mass in a large cylindrical container (you can use a plastic cup) and press a hole into a small one, leaving the bottom thick enough.
As a result, a vessel will come out, which is given time to dry. In this case, heat treatment will also be required to remove excess liquid. If all the steps were correct, then you will have a high-quality graphite crucible, made by yourself.
Making a crucible for melting lead with your own hands
To give the prepared mass the required shape, you can choose one of the options presented below. They look like this:
- use a collapsible or destructible plaster mold;
- blind yourself.
Which option to choose is up to you. The last stage of creating a crucible must be performed strictly according to the specified algorithm:
- After the vessel has been formed, it must be dried.
- As soon as it dries, place the workpiece in a muffle furnace. Set the temperature to 800 degrees and bake for one or two hours. This temperature is necessary so that the glue can melt and firmly bind all other components together. If the temperature is lower, the crucible will fall apart during the first operation in the furnace due to the temperature of the contents. If the temperature is higher, it will fall apart before the end of firing.
View gallery
The resulting form can withstand heat of 1600 degrees. If the materials have been properly processed and milled, it will last up to 30 meltings.
Graphite crucible
Graphite is a material that has many unique properties. Positive qualities of graphite:
- resistance to molten metals;
- increase in strength with increasing temperature;
- high heat resistance and thermal conductivity;
- small specific gravity.
To make a crucible from this material you will need:
- graphite powder;
- solid graphite;
- felt;
- graphite tube;
- fireclay mortar;
- magnesite.
Some of these materials can be used as independent units. For example, a graphite tube is essentially already a crucible; you just need to make a bottom in it.
The principle of manufacturing from all materials is the same. Let's look at the example of mortar. Two forms are made. You can roll it out of thick paper to make it easier to remove later. The outer shape is a hollow cylinder configuration, while the inner one is just a cylinder. The small cylinder is inserted into the wider one. The mixture will be poured between them. The mold is placed in a plastic cup and mortar powder is poured into it. You need to fall asleep with a slide, as it will sit down when you need to compact it. 15 cubes of liquid glass are poured into this powder using a syringe. Everything is mixed and the consistency of shortcrust pastry is obtained. Stuff into the mold in small portions.
The result is something like a glass turned upside down. To prevent the form from sticking to the table, it is best to do the entire procedure on cellophane. Then the mold is turned upside down and the inner cylinder is removed. It is also best to initially glue it with cellophane or tape. Then, when removing, the shape of the crucible will not be damaged.
After the crucible has dried, it must be placed in the inductor and heated. This must be done at low temperatures, since all the water should evaporate, despite the fact that outwardly it seems as if it is not there at all. If the crucible is not preheated and you immediately start melting in it, it will most likely burst. After warming up, when you tap the crucible, it will make a ringing sound. This indicates that the crucible is well made.
By following the instructions presented, you can quite easily acquire a homemade melting furnace that will last no less than a purchased one. The main thing is to take your time, be careful in your work and not violate manufacturing technologies.
In this article I will tell you how to make a crucible with your own hands.
Among the metals, I have successfully melted aluminum, copper, silver and various alloys in it.
Step-by-step instructions for melting copper
Copper smelting, if you prepare everything necessary for the implementation of such a technological process and approach its implementation correctly, allows you to produce copper products for both decorative and purely practical purposes even at home.
To melt copper, you will need the following tools, equipment and supplies:
- muffle furnace (preferably with adjustable heating temperature);
- a crucible in which you will melt copper (for melting copper, crucibles made of ceramics or refractory clay are used);
- tongs with which the hot crucible will be removed from the furnace;
- hook (it can be made from ordinary steel wire);
- household vacuum cleaner;
- charcoal;
- the mold into which the casting will be performed;
- gas burner and forge.
Electrical copper contains the least amount of impurities
The crushed copper is placed in a crucible. Keep in mind: the smaller the pieces of metal, the faster it will melt. After filling the crucible with copper, it is placed in a furnace, which, using a temperature regulator, must be heated to the required state. The doors of serial muffle furnaces must have a window through which you can observe the melting process.
The viewing window will allow you to control the process without opening the door again, thereby not reducing the temperature in the oven
After all the copper in the crucible has melted, it must be removed from the furnace using special tongs. There is always an oxide film on the surface of the molten copper, which must be moved to one of the walls of the crucible using a steel hook. After freeing its surface from the oxide film, the molten metal should be poured as quickly and carefully as possible into a pre-prepared mold. The details and rules for performing this procedure are well demonstrated in a video that is easy to find on the Internet.
You will have to pour the metal into molds very quickly if the heating method you chose could not provide the desired temperature
If you do not have a muffle furnace at your disposal, you can heat the crucible with copper using a gas burner, placing it vertically under the bottom of the container
In this case, it is important to ensure that the flame of the gas burner is evenly distributed over the entire area of the bottom of the crucible
If at home you need to melt low-melting alloys based on copper (brass and some brands of bronze), then you can use a regular blowtorch as a heating device, also placing it vertically under the bottom of the crucible. When melting is performed using this and the previous methods, the surface of the molten metal will actively interact with oxygen, which will lead to intense oxidation. To reduce the intensity of oxidation, molten copper can be sprinkled with crushed charcoal.
Melting copper with a blowtorch in a homemade stove
If you have a forge in your home workshop, it can also be used to melt copper, brass or bronze. In this case, a crucible with crushed metal is placed on a layer of hot charcoal. To make the heating and melting process more intense, air can be supplied to the coal combustion zone, for which a regular vacuum cleaner that works not to draw in, but to blow out is suitable. If you use a vacuum cleaner, you need to make a metal tip with a small diameter blowing hole on its hose.
The smelting process will be even more efficient in a gas furnace
When selecting a muffle furnace for performing foundry operations with copper and its alloys, you should pay attention to the temperature conditions that such a device can provide. Depending on the type of metal being melted, such a furnace must provide the following heating temperatures:
- copper – 1083°;
- various grades of bronze – 930–1140°;
- brass – 880–950°.
It is possible that you will decide to make a smelting furnace yourself after watching the video.
Ordinary copper, which does not contain any alloying additives in its chemical composition, does not have good fluidity in the molten state, therefore it is not suitable for the manufacture of casting products of complex configurations and small sizes. For these purposes, it is best to use brass, and choose an alloy whose surface color is lighter (this indicates that brass of this brand has a lower melting point).
Making a crucible furnace
The easiest way to make a crucible is to simply weld it from a piece of pipe of the most suitable diameter. When choosing the trim itself, you need to consider several important factors.
First, the width of the walls should be at least half a centimeter. Second, your scrap must be made of a metal that melts at a higher temperature than the alloys you plan to melt. Cast iron is ideal for this. After this, the pipe must be cleaned, or all excess can be simply burned in the oven. Now your homemade crucible furnace is in no way inferior to any other.
To safely remove molten metal from the crucible, attach a small spout to it. To do this, grind the top a little with a grinder and go through it with a file. To make it, use a piece of metal cut at an angle.
You can also attach a handle to your homemade crucible furnace; use a nut and screw the handle into it, which will help you immerse and remove the crucible from the furnace more safely. All you need is a couple of turns of the handle, and your crucible will be held in a death grip, thus preventing accidental tipping or spilling of molten aluminum on yourself or people who may be nearby at that moment. All the safety measures mentioned above will come in very handy when melting non-ferrous metals at home. As you can now see, installing a crucible furnace with your own hands is a fairly simple task. I hope you will now never ask yourself again: how to make a crucible furnace?
Crucible markings
Crucible (VIII-IX centuries). Archaeological excavations of the 1970s, Kamno settlement, Pskov region. State Museum of the History of St. Petersburg
Each crucible has a brand (number), and this number indicates the capacity of the crucible. Crucibles are marked from 1 to 300. In metallurgy, as the most widespread industry that consumes crucibles, one conventional unit of capacity (1) is taken to be a volume equal to 0.142 dm³ (or the specific capacity of 1 kg of bronze), provided that the crucible is filled with metal to 85 %. For example, the capacity of a crucible of grade 20 corresponds to 2.84 dm³, or, in other words, 20 kg of molten bronze.
Material selection
Here, fire-resistant components play a serious role, which include:
- Ceramics is an average option, perfect for personal use. There are no reactions that can change the structure of the metal in this cookware, and it is excellent for cobalt, chromium and palladium.
- Clay is a substance that is used in the production of crucibles for jewelers. This component is highly fire-resistant and can withstand up to +1600 °C. If a person wants to create jewelry in his own room, but does not know what to use for melting, then this option is definitely the best.
- Graphite is excellent for melting galvanized and brass alloys, and its main advantage is durability. As for the operating temperature, it should not exceed +800 °C.
- Cast iron. Crucibles made from this substance are rare and belong to the budget categories. Also, products of this kind will have disadvantages in the form of rapid oxidation, low heat resistance and fast production (up to 30 heats).
Varieties of homemade crucibles
As an alternative, you can use an electric crucible, which you can do yourself without much difficulty. It has several uses, but the main one is gold refining.
LiveInternetLiveInternet
Many craftsmen make crucibles for melting metals with their own hands, they have perfected their technologies, and the quality of such products is quite good. If making a crucible with your own hands is a new thing for you, then this review will help you decide which path to take next: make the crucible yourself or buy it from a direct manufacturer without intermediaries.
Let's start with the fact that the materials for making a crucible with your own hands can be pieces of stainless steel pipes, cast iron, clay, fireclay chips, charcoal, graphite, etc. It would seem that the materials are cheap, the cost of a homemade crucible should also be low. However, in practice, when making a high-quality and cheap crucible at home, craftsmen are faced with a number of problems. In this review we will not dwell on graphite crucibles and their burnout problems when melting metals. Here we will dwell in more detail on the problems of making ceramic crucibles with our own hands. I will describe the most common of them:
- The main problem is the heterogeneous composition of the clay masses. Different clay deposits are characterized by different compositions, and in order to obtain high-quality ceramic products, clays of a certain qualitative and quantitative composition are required.
- Clays used to make ceramic crucibles shrink; different clays shrink differently. If you prepare crucibles in large batches and purchase one type of clay in large quantities, then this is not a problem. The shrinkage of the clay is calculated in advance, and the finished product is obtained in a given shape and the variation in overall dimensions is extremely small. But if ceramic crucibles are made from case to case, different batches of clay are used, then maintaining the required dimensions turns out to be problematic, and the percentage of defects increases.
- Homemade ceramic crucibles can withstand a small amount of melting; when using sodium tetraborate (borax), homemade ceramic crucibles quickly become covered with microcracks and become unsuitable for further melting of metal.
- Homemade crucibles are often not sufficiently dried, which is why, when the metal melts, additional stress appears in the body of the crucible and the crucible bursts.
- Homemade ceramic crucibles are thick-walled and require more heating time; energy consumption increases when melting metal.
The best option for melting most metals is a ceramic crucible, but not every ceramic crucible is suitable for melting metal. Let's consider this issue in more detail. Let's start with the fact that ceramic crucibles, which are most often found on the Russian market, are divided into porcelain, corundum and quartz ceramic crucibles. Porcelain crucibles are not suitable for melting many metals due to their relatively low fire resistance
Corundum crucibles are fire-resistant and durable, but when melting metal there is one very important limitation: the corundum crucible cannot be sharply heated and cooled, the cooling rate of the corundum crucible is 2-3 °C/minute. For melting metal under changing temperatures, a crucible made of sintered quartz ceramics is suitable.
A crucible made of quartz ceramics is inert towards metals, fireproof, chemically resistant, and can withstand sudden temperature changes. For an inexperienced person, making a high-quality crucible from sintered quartz ceramics will require unreasonably large amounts of time, material and energy for experimental work. Thus, for a beginner it is much more profitable not to make a crucible for melting metals with your own hands, but to buy it directly from the crucible manufacturer. Prices for quartz ceramic crucibles can be found here (price list).
Part 1 - Ceramic crucibles and mortars - how to choose the right one Part 2 - Porcelain crucible: purpose, advantages and disadvantages ... Part 4 - Mortar and pestle - where to buy without extra charge and how to choose Part 5 - Production of quartz ceramics Part 6 - Crucible for melting metal – why shouldn’t you do it yourself? Part 7 - Price of crucibles made of quartz ceramics of our production (price list) Part 8 - Chinese crucibles: pros and cons. Let's understand the nuances.
Refractories > HIGHLY REFRACTORY CRUCCILES FOR MELTING METALS AND ALLOYS
| Scientific and technical specialists have developed and produced highly refractory ceramic melting crucibles for various technological processes that meet the demands of modern metallurgy. Crucibles are designed for melting heat-resistant nickel alloys, alloy steels, beryllium, cast iron, non-ferrous metals, their alloys, etc. The main melting units in which crucibles are used are: vacuum induction furnace, induction furnace and resistance furnaces. The quality of the metal obtained during the smelting process directly depends on the quality and correct choice of the ceramic crucible. Based on this, very high demands are placed on crucibles. They must have a high level of erosion resistance without collapsing, withstand temperature changes, and not interact with the melt. At the Bakor Scientific and Technical Center CJSC, crucibles are manufactured using various methods from ceramic masses based on highly refractory fused materials: periclase, corundum, mullite-corundum, aluminum-magnesium spinel, zirconium dioxide, which ensures high heat resistance and corrosion resistance of the crucibles, as well as cleanliness melt at temperatures up to 1750-1800 C. The basis for choosing the composition of the crucible and the technology for its production are the conditions of its operation: temperature, contact medium, type of melting plant, etc. The main advantages of ceramic crucibles from STC “Bakor” are: • high level of thermal and erosion resistance; • minimal interaction of the crucible material with the melt; • increasing the yield of suitable castings by reducing rejects of oxide and slag inclusions; •wide selection of products by size; The production of crucibles or additional parts for them according to individual sizes is carried out in accordance with the Customer’s drawings. Continuous improvement of compositions and production technology at the Bakor Scientific and Technical Center CJSC allows us to select and develop products in accordance with technical specifications, based on the Customer’s operating conditions. Our crucibles are supplied to such enterprises as: OJSC "Turbodetal" (Narofominsk, Moscow region), OJSC "Asha Metallurgical Plant", (Asha, Chelyabinsk region), OJSC "Machine-Building Plant" (Elektrostal, Moscow region) and many others. Standardly produced crucibles correspond to the most popular standard sizes (see table SHAPE AND SIZES): |
TU 1501-159-11773998-2014
| Physico-chemical parameters, % mass/grade | ||||
| Indicator name/brand | TK | TP | TCM | TKMC |
| Al2O3,% not less | 97 | — | 90 | 86 |
| MgO,% not less | — | 96 | — | — |
| Open porosity, % no more | 20 | 20 | 20 | 21 |
| Apparent density, g/cm3, not less | 2,90 | 2,85 | 2,80 | 3,00 |
| Ultimate compressive strength, MPa, not less | 40 | 40 | 40 | 40 |
| Application temperature, °C, max | 1750 | 1800 | 1700 | 1700 |
SHAPE AND SIZES
The volume of crucibles is from 1.3 l to 100 l. It is possible to manufacture crucibles according to customer drawings
| Product name and code | Crucible volume, l | Crucible weight, kg (approximate) | Standard sizes | ||||
| D | H | d | h | d days | |||
| №0 | 1,36 | 1,7 | 124 | 200 | 110 | 193 | 50 |
| №1 | 2,6 | 3,7 | 140 | 286 | 120 | 265 | 60 |
| №2 | 4,5 | 8,4 | 168 | 330 | 148 | 307 | 60 |
| №3 | 6,1 | 8,9 | 177 | 405 | 152 | 380 | 60 |
| №18 | 0,9 | 1,2 | 90 | 180 | 76 | 173 | 46 |
| Product name and code | Crucible volume, l | Crucible weight, kg (approximate) | Standard sizes | |||
| D | H | d | h | |||
| №4 | 5,3 | 8,2 | 165 | 370 | 140 | 350 |
| №5 | 5,4 | 8,8 | 195 | 260 | 170 | 240 |
| №6 | 12,1 | 19,5 | 242 | 390 | 206 | 365 |
| №7 | 19,0 | 34,0 | 285 | 465 | 235 | 445 |
| №11 | 93,0 | 187,0 | 560 | 600 | 480 | 560 |
| Product name and code | Crucible volume, l | Crucible weight, kg (approximate) | Standard sizes | |||||
| D | D1 | H | d | d1 | h | |||
| №8 | 3,4 | 5,6 | 165 | 140 | 290 | 138 | 100 | 265 |
| №9 | 2,8 | 7,5 | 230 | 220 | 135 | 190 | 180 | 115 |
| №10 | 20,0 | 32,8 | 330 | 330 | 380 | 280 | 250 | 350 |
| №12 | 3,3 | 3,7 | 148 | 138 | 260 | 128 | 98 | 245 |
| №13 | 7,8 | 14,2 | 197 | 197 | 390 | 165 | 127 | 368 |
| №14 | 7,4 | 10,2 | 210 | 210 | 320 | 186 | 80 | 296 |
| №15 | 10,4 | 20,6 | 230 | 230 | 420 | 185 | 140 | 395 |
| №16 | 70,0 | 128,0 | 500 | 460 | 600 | 420 | 380 | 560 |
| №17 | 6,4 | 14,2 | 147 | 130 | 500 | 127 | 110 | 490 |
| №19 | 4,4 | 8,1 | 178 | 164 | 304 | 151 | 132 | 280 |
| №20 | 3,3 | 6,7 | 175 | 164 | 240 | 147 | 132 | 216 |
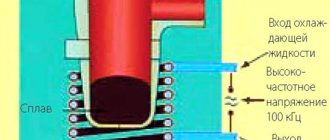
Types of induction furnaces
In the group of metallurgical production equipment, several types of furnaces can be distinguished:
- Crucible.
One of the most common types in metallurgy.
There is no core in the design of such units. Such devices can be used for melting and processing any metals. They have proven themselves well not only in metallurgy, but also in other industries, for example, in jewelry.
The most important elements of an induction crucible furnace are:
- inductor;
power supply generator.
- Energy release directly into the load, without intermediate heating elements;
- Intense electrodynamic circulation of the melt in the crucible, ensuring rapid melting of small batches and waste, equalization of temperature throughout the volume of the bath and the absence of local overheating, guaranteeing the production of multicomponent alloys that are homogeneous in chemical composition;
- The fundamental possibility of creating any atmosphere (oxidizing, reducing or neutral) in the furnace at any pressure;
- High performance achieved thanks to high power densities, especially at mid frequencies;
- The possibility of completely draining the metal from the crucible and the relatively small mass of the furnace lining, which creates conditions for reducing the thermal inertia of the furnace due to a decrease in the heat accumulated by the lining. Furnaces of this type are convenient for periodic operation with breaks between melts and provide the ability to quickly switch from one grade of alloy to another;
- Simplicity and ease of furnace maintenance, control and adjustment of the smelting process, ample opportunities for mechanization and automation of the process;
Advantages of crucible melting furnaces:
Duct.
The design resembles a transformer.
Vacuum.
They are used when it is necessary to ensure the removal of impurities from the melt.
The design of an induction heater is a multi-turn cylindrical coil, which is called an inductor; an alternating current electric voltage is passed through it, resulting in magnetic fields that excite eddy currents.
A vessel or container containing metal or ore is placed in the internal space of the inductor. Under the influence of a magnetic field and eddy currents, the resistance in the metal increases, which, according to all the laws of physics, causes it to heat up and, due to this, the melting process occurs.
The power of induction melting furnaces depends on the magnitude of the applied voltage and the frequency of the electric current. This relationship is used in the types of induction furnaces - heat treatment heating units and melting furnaces.
Industrial furnaces are divided into several types.
- Medium frequency designs are commonly used in mechanical engineering and metallurgy. With their help, steel is melted, and when using graphite crucibles, non-ferrous metals are melted.
- Industrial frequency designs are used in iron smelting.
- Resistance structures are intended for melting aluminum, aluminum alloys, and zinc.
The induction furnace is widely used in large and small enterprises for melting metals (non-ferrous and ferrous). In induction casting furnaces, a metal or alloy is heated until its state of aggregation changes.
At the same time, channel furnaces, despite their higher efficiency, are used much less frequently - mainly for the production of high-quality cast iron and alloys, the melting point of which is relatively low, as well as for the melting of non-ferrous metals.
Such furnaces are not used for steel, since its melting temperature greatly reduces the durability of the lining (protective finish). Also, you cannot melt low-grade rock, shavings and small rock.
Crucible furnaces are used much more often due to their ease of operation and greater process control capabilities, including the possibility of irregular and intermittent operation. They are good both for the production of large quantities of castings of several tens of tons, and for small portions measured in tens of grams.
Crucible furnaces are used to melt alloy steels and other alloys that require special purity of the chemical composition and homogeneity.
Physical features of melting homogeneous metals
Brass is a multicomponent alloy based on copper and zinc. It may also contain some other components - tin, lead, iron, nickel, manganese. Copper acts as the main substance, while additional components improve the physical properties of the material (strength, elasticity, electrical conductivity, corrosion potential). Melting of single-component and multi-component alloys has many differences. Therefore, before considering the issue of melting brass, it is necessary to consider the features of melting a homogeneous metal based on copper.
In physics, smelting is a procedure in which a solid metal turns into a liquid state. To melt copper, it must be heated to a temperature of 1.085 degrees Celsius. Typically, heating is carried out with a small temperature increase (~1150 degrees), since in practice copper alloys with the addition of alloying substances are often used, which increases the melting point.
Heating at the chemical-physical level
- Copper atoms are in a solid state before heating. On a chemical level, this means that they form a strong crystal lattice that is resistant to deformation and retains its shape upon impact.
- When heated, the potential energy of copper atoms increases, which leads to a deterioration in the strength of the crystalline structure of the material. However, the material retains its hardness because the crystal lattice is not destroyed (although it becomes less dense).
- When the temperature reaches 1.085 degrees, the copper atoms receive an excess amount of energy, which causes the crystal lattice of the alloy to disintegrate. At the physical level, the alloy changes from a solid to a liquid state.
- Now several situations are possible. Let's consider the first situation. If the material continues to be heated, it will retain its liquid state. At a temperature of 2.567 degrees, copper goes into a gaseous state (that is, the liquid begins to boil). In metallurgy, evaporation of copper is very rarely performed, since it has no practical benefit.
- But another situation is also possible. If liquid copper is not heated after melting, then the liquid will gradually begin to cool. This will cause the material to return to a solid form. At the chemical level, the crystal lattice will re-form.
One simple conclusion can be drawn from these theoretical calculations. For one-component compositions, the crystallization temperature and melting temperature are the same. In practice, it is simple to regulate the melting procedure - you just need to reduce or increase the temperature of the fire. During work, it is also necessary to monitor the distribution of fire over the entire area of the metal object. If the temperature distribution is uneven, some components will be in a liquid state, while others will be in a solid state.
Making a crucible from clay
You can make a crucible from fireclay clay. This is an inexpensive option and also highly resistant to high temperatures. This clay is used for laying stoves and can be purchased at any hardware store. Fireclay clay can withstand temperatures up to 1600 degrees Celsius.
So, you will need fireclay clay (sold in bags in hardware stores), liquid glass (sold there) and ground fireclay. It can be bought or made from fireclay bricks.
In order to make a mixture from which a crucible will be fashioned in the future, take 7 parts of clay, 3 parts of fireclay and 10 tablespoons of liquid glass per liter of dry mixture. Fireclay and clay are mixed until smooth. After this, water is slowly added. In order not to spoil the workpiece, you can pour out part of the mixture, and if there is a large amount of water, add dry powder. You need to knead until the clay stops sticking to your hands.
Only after the clay of the desired consistency has been mixed can glass be added. When adding glass, you need to thoroughly knead everything until the clay stops cracking. It is best to add glass to a lump of clay and roll it into a roll, then fold it several times and repeat the procedure until it stops cracking. The material for the crucible is ready. Until the moment when it is used, it must be stored in several layers of cellophane.
There is clay, now to make a crucible you need to take a mold, the easiest way is to use a plaster mold. How to make such a form can be found on any website on plaster modeling. So, directly making the crucible.
Before you start sculpting, you need to knock all the air out of the clay; to do this, you can lay a newspaper on the floor and forcefully throw a lump on it several times, ten times will be enough. Now take a lump of clay and carefully press it into the bottom of the mold, after which the walls of the product are formed in small lumps. Their thickness can be controlled along the edge of the mold
It is very important to carefully press the clay into the mold so that no air cushions form there. After the crucible is sculpted, you need to make the inner surface smooth. To do this, simply moisten the clay with water.
To do this, just moisten the clay with water.
After this comes the moment of drying. The mold with clay is placed in a cardboard box and covered with a lid. After seven hours, all the water from the clay will evaporate and the shape of the future crucible will “shrink” a little, so getting it out of the mold is not particularly difficult. After this, the crucible continues to dry in the same box; as it dries, all defects will be eliminated by themselves and the pot will acquire a gray color. Sometimes small cracks may appear. They can be covered with wet clay. Next, the pots are fired at a temperature of 800 degrees in a muffle furnace. After firing, the crucible is ready for use.
Making a crucible furnace
The easiest way to make a crucible is to simply weld it from a piece of pipe of the most suitable diameter. When choosing the trim itself, you need to consider several important factors.
First, the width of the walls should be at least half a centimeter. Second, your scrap must be made of a metal that melts at a higher temperature than the alloys you plan to melt. Cast iron is ideal for this. After this, the pipe must be cleaned, or all excess can be simply burned in the oven. Now your homemade crucible furnace is in no way inferior to any other.
To safely remove molten metal from the crucible, attach a small spout to it. To do this, grind the top a little with a grinder and go through it with a file. To make it, use a piece of metal cut at an angle.
You can also attach a handle to your homemade crucible furnace; use a nut and screw the handle into it, which will help you immerse and remove the crucible from the furnace more safely. All you need is a couple of turns of the handle, and your crucible will be held in a death grip, thus preventing accidental tipping or spilling of molten aluminum on yourself or people who may be nearby at that moment. All the safety measures mentioned above will come in very handy when melting non-ferrous metals at home. As you can now see, installing a crucible furnace with your own hands is a fairly simple task. I hope you will now never ask yourself again: how to make a crucible furnace?
Crucible markings
Crucible (VIII-IX centuries). Archaeological excavations of the 1970s, Kamno settlement, Pskov region. State Museum of the History of St. Petersburg
Each crucible has a brand (number), and this number indicates the capacity of the crucible. Crucibles are marked from 1 to 300. In metallurgy, as the most widespread industry that consumes crucibles, one conventional unit of capacity (1) is taken to be a volume equal to 0.142 dm³ (or the specific capacity of 1 kg of bronze), provided that the crucible is filled with metal to 85 %. For example, the capacity of a crucible of grade 20 corresponds to 2.84 dm³, or, in other words, 20 kg of molten bronze.
Casting shape
If you only need to cast pure aluminum for solder, then a casting mold is not needed. It is enough to use a steel sheet on which the molten metal will cool. But if you need to cast even a simple part, you will need a casting mold.
The casting mold can be made from sculptural plaster, namely gypsum, not alabaster. Liquid plaster is poured into an oiled mold, allowed to harden a little, shaking occasionally to release air bubbles, the model is inserted into it and covered with a second container of plaster. In a convenient place, you need to insert a cylindrical object into the plaster so that eventually a hole appears in the mold, the so-called channel, into which molten aluminum will be poured. When the plaster has completely hardened, the two parts of the mold are separated, the model is taken out, and the mold with the finished cast is connected again.
A casting mold can also be made from a mixture of 75% foundry sand, 20% clay and 5% coal sand, which is poured into a special box made of boards and compacted. The model is pressed into the compacted earth, the resulting imprint is sprinkled with talcum powder and graphite (coal dust) so that the cooled aluminum part can be easily separated from the mold.
Read also: Which hiller is best for a walk-behind tractor?