Gold-plated products are popular due to their brightness and expensive appearance, at a relatively low price relative to gold products. If stored improperly or frequently used, the product gradually loses its performance characteristics: it darkens and fades. Keeping your product clean and tidy is easy if you use the tricks and secrets of cleaning and care. This article contains the most effective and proven methods for cleaning gold plating at home.
Cleaning Rules
A gold-plated item is different from silver or gold, so the methods used for them are absolutely not suitable. Gold plating is very easy to ruin. The first stage of cleaning is the removal of surface dirt - dust, stains, grease. To do this, it is enough to treat the surface with alcohol or turpentine.
Gold plating is soft, so it is not recommended to use strong mechanical impact, as there is a risk of damage to the surface. Suede fabric is excellent for care; it adds shine to products and removes stains. To clean a gold-plated watch, sometimes it is enough to treat it with suede. If you need to clean gold-plated silverware, remove any grease from it first.
Before processing, perform the following steps:
- Take a clean, dry cloth and wipe the gold with it. Flannel, suede and wool are perfect for this.
- Then remove surface dirt.
What hazards do chemical reactions and processes entail?
Naturally, you need to understand that any work with acids can have serious consequences.
For example, working with aqua regia, or rather the refining process where metals are dissolved, takes about six hours.
In this case, a strong release of nitric oxide occurs.
Therefore, in no case should such work be carried out in closed lighting; the windows must be open so that fresh air has a constant opportunity to enter the room.
One breath of poisoned air with oxides can cause a sudden and painful death, since doctors simply do not have time to provide first aid.
Methods for removing stains from gold plated
To clean gold plating at home, you need to select the product carefully so as not to harm the capricious surface. If the dark spots are in a small area and are not very ingrained, then at home it is enough to polish with a cloth. If the item is heavily soiled or has been stored for a long time without care, an effective cleaning method will be required.
Wine spirit
Gold-plated items can be easily cleaned from tarnish and dirt at home using wine alcohol. Saturate a soft sponge with the product and treat the surface of the product. You can replace alcohol with wine vinegar, which can be found in the kitchen. At the final stage, treat the surface with suede to rub the surface to the desired shine.
Cleaning with beer
You can clean a gold-plated chain, earrings, ring and other items dusted with gold at home using beer. Oddly enough, the mild action of this alcoholic drink effectively copes with darkening of the coating and any dirt, eliminating damage to the material.
Pour beer into a glass, dip the gold-plated item into it and leave for 30 minutes, then rinse thoroughly and wipe dry.
Soap solution
Take any hard soap and grate it on a coarse grater. Take 1 tsp. shavings and dilute in 1 liter. warm water. For best results, use a couple of drops of ammonia added to soapy water. Immerse the gold in the container for 30 minutes, rinse with cool running water, and wipe dry with a clean cloth. The product effectively fights stains that have a green tint.
Alcohol and turpentine
The products help fight grease stains, darkening and dirt on the surface of gold-plated products. Ethyl, medical, denatured alcohol is suitable for cleaning. Take one of the products available in the house, saturate a soft sponge with it, treat the gilding, rinse off any remaining dirt and product under running water, and polish with a cloth.
Table vinegar
The diluted product is used to wipe or soak the product.
- Stir 2 tbsp. acids in 1 tbsp. water, moisten the sponge and treat the surface with the composition.
- Stir 2 tbsp. acids in 1 l. water and place the item in the composition for 15 minutes.
After all procedures, it is important to rinse the gold plating from any remaining product and dry it thoroughly. Acid residue can destroy the gold plating and permanently damage your favorite jewelry or cutlery.
Egg white against darkening
Break the egg and separate the yolk from the white. In this recipe we need protein. Dip a cotton pad into the white and apply it to the gilding. Cleaning will restore the shine and brightness of the coating. The effectiveness of the product will be higher if you add a tablespoon of white to the protein. When working with chemicals, you will need to protect your hands with gloves.
Method for extracting gold from radio components without heating
If you are puzzled by the question: how to extract gold without dangerous work? This method also works, however, it takes much longer, a week. Parts containing gold are dipped into a liquid that consists of hydrochloric acid and hydrogen peroxide in a ratio of 2 to 1.
In 7 days, gold is separated from other metals. Then you need to pass the solution through a filter, you can use a paper one, and wash the precipitate with methyl alcohol. The residue is also dried and melted into an ingot.
There are other methods for extracting precious metals, but they are much more complex and dangerous than those described above. Gold can also be obtained using electrolysis, sulfuric and hydrochloric acids.
To get rid of unwanted particles, you need to carry out a procedure called refining. This is a more detailed and fine cleaning using acids, resulting in the extraction of pure gold.
What can't you do to clean gold plate?
Any products used should not contain abrasive particles. Even the smallest particles can severely damage the spray. For example, you should not clean gold plate with chalk, toothpaste, soda or powder.
Any abrasive product will cause scratches.
Some products are coated with a very thin layer of coating; abrasives easily erase it. Do not use rough scourers or brushes, or aggressive acids for cleaning.
Prevention and care rules
The gold plating wears off over time. Constant use leads to a gradual thinning of the spray layer. By following some recommendations you can extend the service life. Rules for caring for gold-plated products:
- Regularly polish cutlery and decorations using a suede cloth.
- Minimize contact with water, perfume and body creams.
- Place each product separately, so that they do not touch each other. After each use of jewelry, put it back in place. Distribute each decoration into separate suede bags, and cutlery into special boxes.
Clean regularly, regardless of whether the product has lost its attractive appearance or not.
Cutlery made of cupronickel, as well as silver, is found in almost every home. So I, while cleaning out my parents’ closets, came across a small box with teaspoons made of cupronickel with gold plating. But after lying unnecessarily for thirty years, they acquired a very deplorable appearance.
In search of information on how to clean cupronickel spoons, I looked through a lot of different sites and found many fairly simple ways to eliminate this terrible coating on spoons. In practice, I decided to try several cleaning options using simple home remedies as an experiment.
To begin with, I tried to simply wash the spoons using my usual dishwashing detergent, but this, of course, had no effect on the plaque.
So we'll experiment. 1 way. Cover the bottom of a stainless (or enamel) container with foil and place spoons. Pour in 60 g of soda ash and 40 g of salt.
Fill with hot water so that the objects are completely covered with it. Place the container on the stove and boil for 15 minutes over low heat (so that the signs of boiling are barely noticeable). The spoons begin to lighten right before our eyes.
We rinse them with clean water to remove traces of soda and salt, and wipe them dry with a soft cloth. There was no trace left of the black spots.
This cleaning method is not recommended if nickel silver items have gold plating. But I still took the risk. The gilding has become somewhat lighter, but this is not a problem.
Method 2. Apply a little toothpaste to a cotton pad (you can also use powder) and rub the nickel silver spoon, of course, with a little effort. And the spoons sparkled again with their original shine. What an effect!
3 way. Pour a liter of water into a saucepan, put it on fire, throw in the crushed shells of two eggs (raw) and 30 g of salt.
As soon as the water boils, we place cupronickel spoons in it, boil for literally two minutes, and... all efforts were in vain. The spoons have not changed at all.
Method 4 is intended specifically for nickel silver with gilding. Wet a ball of cotton wool with apple cider vinegar (you can also use egg white, turpentine or wine vinegar) and rub the spoon with it thoroughly. Alas! Again no positive result. I even tried to soak the spoon in a pure vinegar solution for several minutes, but to no avail.
5 way. Grate the peeled potato tuber on a fine grater and dip cupronickel spoons into the pulp.
Perhaps this cleaning method is the safest, since there is no need to rub or boil, much less use chemicals. Only natural ingredients. True, there is no sense in it either. All the blackness remained in place.
Here are 5 ways I've tried to clean cupronickel silver cutlery. What I liked most was the option using toothpaste. It was with this that I cleaned all the spoons that took part (to no avail) in other experiments. The paste instantly removes all the blackness.
Removing gilding with electrolyte and ammonium nitrate
To prepare a solution that will help remove gold plating from radio components, you will need an electrolyte for car radiators and ammonium nitrate.
Sell radio components
A small hole of 3-5 mm is made in the lid of a half-liter nylon mayonnaise container and 200 to 300 ml of electrolyte is poured into the jar. Then add 100 grams of saltpeter and gently stir the mixture until the saltpeter dissolves.
Gold-plated parts weighing no more than 100 grams are immersed in the solution and covered with a lid with a hole, after which the container is heated in a water bath in a pan of boiling water and then the solution is continued to boil.
Due to the chemical reaction that has begun, hissing will begin and a brown gas will begin to be released through the lid. After this, the bucket with the solution can be removed from the pan of boiling water and cooled.
The result of this procedure will be gold plating that floats to the surface in the form of films. Using a cloth folded several times, the solution, previously diluted with water, is filtered, and the resulting gold particles are dried.
A little history
Cupronickel as such does not exist in nature: in fact, it is a group of compositions made of copper with the addition of nickel, iron, zinc, manganese and some other metals. Most often it is an alloy of copper and nickel, which has its own specific features and properties. According to historical sources, it was invented in China in the 8th century BC and given the sonorous name “pakfong”. All attempts to unravel the composition of “Chinese silver” by European alchemists were unsuccessful: only by the beginning of the 19th century did mass production of nickel silver begin in Germany. At first, the European nobility considered such dishes to be “the dishes of the poor,” and therefore still preferred dishes made of other metals - gold and silver. Only by the 20th century did production increase its speed everywhere. The attractive appearance of cupronickel was used to make cutlery, candlesticks, jewelry and other decorative elements. Wear-resistant, easy to maintain and not too expensive, the alloy is appreciated and actively used in household use.
Interestingly, psychologists consider cupronickel tableware to be beneficial for the psyche. The unobtrusive, calm, noble shine of the alloy calms the nervous system, relieves tension and sets you to a calm rhythm.
What parts contain gold?
Both silver and gold can be equally used to coat radio components. But despite the greater electrical conductivity of silver and the lower degree of electrical resistance of silver, manufacturers prefer gold. This is due to the fact that this precious metal oxidizes much more slowly, which allows the parts to serve for many years.
Foreign manufacturers also use gold to coat microcircuits, but if you are wondering about this, the answer is that in domestic technology you can get much more gold from radio components.
It is worth noting here that a higher percentage of gold content is found in Soviet-made radio components. Surely you have old parts lying around in the basement, attic or mezzanine that you always felt a pity to throw away. This is where they come in handy.
So, in Soviet electronics: televisions, tape recorders, radios, almost all radio components contained gold in one way or another. What parts are gold plated and where can I get them?
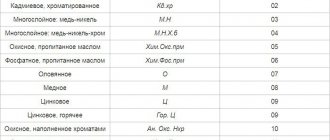
- Transistors whose gold content is located under the crystal and conductor, sometimes on the legs. Basically, these are transistors of the KT series (101,103, 117, 603, 613, etc.).
- Gold can also be mined from microcircuits, because... the precious metal was deposited by galvanic means, i.e. coating one metal with another through the action of electric current.
- Connectors made during the Soviet period are also coated with a layer of gold several microns thick.
- Radio tubes, in addition to gold, they may also contain silver, platinum, tantalum (models: 12P17L, 6V1P, 6Zh1P-EV, GMI-11, etc.) A more detailed description of radio tubes can be found in specialized literature. One cannot help but mention the radio tubes of the GMI series; in some models the weight of pure gold can reach 16 grams.
- In semiconductors, in particular, in D-series diodes, LEDs, zener diodes, thyristors, etc. there is gold in small quantities.
- Capacitors contain the largest amount of gold, but only those that were used only for the construction of military equipment, and of the old type.
- Microparticles of gold are also contained in modern parts, for example, in SIM cards.
- In the manufacture of metal wristwatches in the USSR, the precious metal acted as a coating for the case.
- Computer parts also consist of gold elements: processors, connectors on motherboards, etc. It is important to know that gold extraction will be successful if the computer is “older”, because it contains more precious metal.
How to clean spoons, forks and other dishes made of cupronickel
There are several standard rules for caring for nickel silver products, the implementation of which guarantees shine and cleanliness:
- Wash cutlery regularly with regular dishwashing gel.
- For cleaning, use only soft sponges.
- After washing, wipe the products thoroughly, do not leave moisture on them.
- Rub nickel silver with a microfiber cloth or velvet cloth to add shine.
- It is advisable to store nickel silver dishes in a dry place, wrapped in paper.
How to add shine and remove blackness
Sometimes cupronickel knives and forks lose their former shine and begin to turn black. This is a common drawback of cupronickel alloy. The cause of color loss is most often high humidity. The following methods will help:
- Cleaning with soda. Baking soda is turned into a paste and rubbed onto cutlery. Then they are washed and dried, and if necessary, the procedure is repeated again.
- Using ammonia. The devices are wiped with ammonia and rubbed until shiny with a dry cloth.
- Cleaning with chalk. Chalk and laundry soap shavings are mixed with a liter of water so that a saturated composition is obtained. Cupronickel is placed there and cleaned. After the procedure, everything is rinsed and dried.
- Eggshells will help remove old stains. The shells of two eggs are mixed with a liter of water and boiled. The devices are immersed in the hot solution for 20 minutes, and upon removal, they are rinsed and dried.
- Potato decoction is good for getting rid of blackness. The products are dipped into boiling potato broth for at least a quarter of an hour and then taken out. Rinse everything with clean water and rub until shiny.
- Garlic peel. It is added to boiling water and boiled. Cupronickel silver spoons and forks are placed there, which, when removed after a quarter of an hour, begin to shine.
- Cleaning with food foil. An original and effective method. A sheet of foil is placed at the bottom of the aluminum container, and the cutlery is laid out on it. Pour a baking soda solution over everything and boil. During this time, a reaction occurs, as a result of which the foil picks up contaminants. Upon removal, everything is washed under the tap and rubbed. The products will shine. Such cleaning with foil cannot be carried out with gold and silver plating, as the reaction will inevitably damage it.
Cleaning cupronickel is a simple task that requires a minimum of effort and money. The above cleaning methods are effective and safe. They guarantee high-quality care and long service life of cupronickel cutlery.
Separation of gold plating from radio components using electrolysis
In a porcelain or glass heat-resistant container with a lid, prepare a solution of 1 liter of distilled water and 50 grams of potassium cyanide. Plates made of iron or silver are suitable for the role of the oxidizer (cathode).
Pre-cleaned gold-plated radio components are hung onto another reducing electrode (anode) and lowered into a container with a solution. After this, the DC supply is turned on - 12-15 V. During the reaction, you cannot open the lid, because the fumes released are toxic. The gold will settle on the cathode in the form of a thin film that needs to be scraped off the plate.