As we know from Ohm's law, the current in a section of the circuit is in the following relationship: I=U/R . The law was derived through a series of experiments by the German physicist Georg Ohm in the 19th century. He noticed a pattern: the current strength in any section of the circuit directly depends on the voltage that is applied to this section, and inversely on its resistance.
Later it was found that the resistance of a section depends on its geometric characteristics as follows: R=ρl/S ,
where l is the length of the conductor, S is its cross-sectional area, and ρ is a certain proportionality coefficient.
Thus, the resistance is determined by the geometry of the conductor, as well as by such a parameter as resistivity (hereinafter referred to as resistivity) - this is the name of this coefficient. If you take two conductors with the same cross-section and length and place them in a circuit one by one, then by measuring the current and resistance, you can see that in the two cases these indicators will be different. Thus, electrical resistivity is a characteristic of the material from which the conductor is made, or, to be even more precise, of the substance.
Stainless steel resistivity table
Tables of electrical resistivity values of steels of various types and grades are presented depending on temperature - in the range from 0 to 1350°C.
In general, resistivity is determined only by the composition of the substance and its temperature; it is numerically equal to the total resistance of an isotropic conductor having a length of 1 m and a cross-sectional area of 1 m 2.
The electrical resistivity of steel depends significantly on composition and temperature. As the temperature of this metal increases, the frequency and amplitude of vibrations of the atoms of the crystal lattice increases, which creates additional resistance to the passage of electric current through the thickness of the alloy. Therefore, with increasing temperature, the resistance of steel increases.
Changing the composition of steel and the percentage of alloying additives in it significantly affects the value of electrical resistance. For example, carbon and low-alloy steels conduct electric current several times better than high-alloy and heat-resistant steels, which have a high nickel and chromium content.
Carbon steels
Carbon steels at room temperature, as already mentioned, have low electrical resistivity due to their high iron content. At 20°C, the value of their resistivity is in the range from 13·10 -8 (for steel 08KP) to 20·10 -8 Ohm m (for U12).
When heated to temperatures above 1000°C, the ability of carbon steels to conduct electric current is greatly reduced. The resistance value increases by an order of magnitude and can reach a value of 130·10 -8 Ohm·m.
Electrical resistivity of carbon steels ρe·10 8 , Ohm·m Temperature, °SSteel 08KPsteel 08Steel 20Steel 40Steel U8Steel U12
Low alloy steels
Low alloy steels are able to resist the passage of electricity slightly more than carbon steels. Their electrical resistivity is (20...43)·10 -8 Ohm·m at room temperature.
It should be noted that steel grades of this type are the worst conductors of electric current - these are 18Х2Н4ВА and 50С2Г. However, at high temperatures, the ability to conduct electric current among the steels listed in the table practically does not differ.
AISI 304
International standard American ASTM A240 European EN 10088-2 Russian GOST 5632-72
| Brand designation | AISI 304 | 1.4301 | 08Х18Н10 |
| 12Х18Н9 |
AMS 5513 ASTM A 240
ASTM A 666
Classification
corrosion-resistant, heat-resistant steel
Application
- Household items
- Sinks
- Frames for metal structures in the construction industry
- Kitchen utensils and catering equipment
- Dairy equipment, brewing
- Welded structures
- Tanks on ships and land tankers for food, beverages and some chemicals
Typically, steel manufacturers divide the grade into three main classes (grades) according to their drawing ability:
- AISI 304 - Main grade
- AISI 304 DDQ (Normal and deep drawing) - Deep drawing grade
- AISI 304 DDS (Extra deep drawing) - Extra deep drawing grade
Main characteristics
- good overall corrosion resistance
- good ductility
- excellent weldability
Chemical composition (% by weight)
standard grade C Si Mn PS Cr Ni
| ASTM A240 | AISI 304 | ≤0.080 | ≤0.75 | ≤2.0 | ≤0.045 | ≤0.030 | 18.00 — 20.00 | 8.00 — 10.50 |
Mechanical properties
AISI 304 Tensile strength (σв), N/mm² Yield strength (σ0.2), N/mm² Yield strength (σ1.0), N/mm² Elongation (σ), % Brinell hardness (HB) Rockwell hardness (HRB)
| According to EN 10088-2 | ≥520 | ≥210 | ≥250 | ≥45 | — | — |
| According to ASTM A 240 | ≥515 | ≥205 | — | ≥40 | 202 | 85 |
Mechanical properties at high temperatures
All these values refer only to AISI 304
.
Physical properties
Physical properties Symbols Unit of measurement Temperature Value
| Density | d | — | 4°C | 7.93 |
| Melting temperature | °C | 1450 | ||
| Specific heat | c | J/kg. K | 20°C | 500 |
| Thermal expansion | k | W/mK | 20°C | 15 |
| Average coefficient of thermal expansion | α | 10-6.K-1 | 0-100°C 0-200°C | 17.5 18 |
| Electrical resistivity | ρ | Ωmm2/m | 20°C | 0. 80 |
| Magnetic permeability | μ | at 0.80 kA/m DC or h/h AC | 20°C μ μ discharge air | 1.02 |
| Elastic modulus | E | MPa x 103 | 20°C | 200 |
Corrosion resistance
304 steels have good resistance to general corrosive environments, but are not recommended where there is a risk of intergranular corrosion. They are well suited for use in fresh water and urban and rural environments. In all cases, regular cleaning of external surfaces is necessary to maintain their original condition.
304 steels have good resistance to various acids:
- phosphoric acid in all concentrations at ambient temperature,
- nitric acid up to 65% at temperatures 20°C - 50°C,
- formic and lactic acid at room temperature,
- acetic acid at a temperature of 20°C - 50°C.
They are recommended for the production of equipment in contact with cold or hot food products: wine, beer, milk (fermented milk products), alcohol, natural fruit juices, syrups, molasses, etc.
Acidic environments
Temperature, °C 20 80
| Concentration, % by weight | 10 | 20 | 40 | 60 | 80 | 100 | 10 | 20 | 40 | 60 | 80 | 100 |
| Sulfuric acid | 2 | 2 | 2 | 2 | 1 | 0 | 2 | 2 | 2 | 2 | 2 | 2 |
| Nitric acid | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
| Phosphoric acid | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 2 |
| Formic acid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 0 |
Code: 0 = high degree of protection - Corrosion rate less than 100 µm/year 1 = partial protection - Corrosion rate from 100 to 1000 µm/year
2 = no protection - Corrosion rate more than 1000 µm/year
Atmospheric influences
Comparison of 304
grades with other metals in various environments (Corrosion rate calculated for 10-year exposure).
Environment Corrosion rate (µm/year)AISI 304 Aluminum-3S Carbon steel
| Rural | 0.0025 | 0.025 | 5.8 |
| Marine | 0.0076 | 0.432 | 34.0 |
| Industrial Marine | 0.0076 | 0.686 | 46.2 |
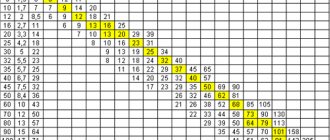
Electrical resistivity of steel at different temperatures
Tables of electrical resistivity values of steels of various types and grades are presented depending on temperature - in the range from 0 to 1350°C.
In general, resistivity is determined only by the composition of the substance and its temperature; it is numerically equal to the total resistance of an isotropic conductor having a length of 1 m and a cross-sectional area of 1 m2.
The electrical resistivity of steel depends significantly on composition and temperature. As the temperature of this metal increases, the frequency and amplitude of vibrations of the atoms of the crystal lattice increases, which creates additional resistance to the passage of electric current through the thickness of the alloy. Therefore, with increasing temperature, the resistance of steel increases.
Changing the composition of steel and the percentage of alloying additives in it significantly affects the value of electrical resistance. For example, carbon and low-alloy steels conduct electric current several times better than high-alloy and heat-resistant steels, which have a high nickel and chromium content.
High alloy steels
High-alloy steels have electrical resistivity several times higher than carbon and low-alloy steels. According to the table, it can be seen that at a temperature of 20°C its value is (30...86)·10-8 Ohm·m.
At a temperature of 1300°C, the resistance of high- and low-alloy steels becomes almost the same and does not exceed 131·10-8 Ohm·m.
| 12 | 13,2 | 15,9 | 16 | 17 | 18,4 | |
| 20 | 13 | 14,2 | 16,9 | 17,1 | 18 | 19,6 |
| 50 | 14,7 | 15,9 | 18,7 | 18,9 | 19,8 | 21,6 |
| 100 | 17,8 | 19 | 21,9 | 22,1 | 23,2 | 25,2 |
| 150 | 21,3 | 22,4 | 25,4 | 25,7 | 26,8 | 29 |
| 200 | 25,2 | 26,3 | 29,2 | 29,6 | 30,8 | 33,3 |
| 250 | 29,5 | 30,5 | 33,4 | 33,9 | 35,1 | 37,9 |
| 300 | 34,1 | 35,2 | 38,1 | 38,7 | 39,8 | 43 |
| 350 | 39,3 | 40,2 | 43,2 | 43,8 | 45 | 48,3 |
| 400 | 44,8 | 45,8 | 48,7 | 49,3 | 50,5 | 54 |
| 450 | 50,9 | 51,8 | 54,6 | 55,3 | 56,5 | 60 |
| 500 | 57,5 | 58,4 | 60,1 | 61,9 | 62,8 | 66,5 |
| 550 | 64,8 | 65,7 | 68,2 | 68,9 | 69,9 | 73,4 |
| 600 | 72,5 | 73,4 | 75,8 | 76,6 | 77,2 | 80,2 |
| 650 | 80,7 | 81,6 | 83,7 | 84,4 | 85,2 | 87,8 |
| 700 | 89,8 | 90,5 | 92,5 | 93,2 | 93,5 | 96,4 |
| 750 | 100,3 | 101,1 | 105 | 107,9 | 110,5 | 113 |
| 800 | 107,3 | 108,1 | 109,4 | 111,1 | 112,9 | 115 |
| 850 | 110,4 | 111,1 | 111,8 | 113,1 | 114,8 | 117,6 |
| 900 | 112,4 | 113 | 113,6 | 114,9 | 116,4 | 119,6 |
| 950 | 114,2 | 114,8 | 115,2 | 116,6 | 117,8 | 121,2 |
| 1000 | 116 | 116,5 | 116,7 | 117,9 | 119,1 | 122,6 |
| 1050 | 117,5 | 117,9 | 118,1 | 119,3 | 120,4 | 123,8 |
| 1100 | 118,9 | 119,3 | 119,4 | 120,7 | 121,4 | 124,9 |
| 1150 | 120,3 | 120,7 | 120,7 | 122 | 122,3 | 126 |
| 1200 | 121,7 | 122 | 121,9 | 123 | 123,1 | 127,1 |
| 1250 | 123 | 123,3 | 122,9 | 124 | 123,8 | 128,2 |
| 1300 | 124,1 | 124,4 | 123,9 | — | 124,6 | 128,7 |
| 1350 | 125,2 | 125,3 | 125,1 | — | 125 | 129,5 |
Electrical resistivity of high-alloy steels ρe·108, Ohm·mSteel grade 2010030050070090011001300
| G13 | 68,3 | 75,6 | 93,1 | 95,2 | 114,7 | 123,8 | 127 | 130,8 |
| G20H12F | 72,3 | 79,2 | 91,2 | 101,5 | 109,2 | — | — | — |
| G21X15T | — | 82,4 | 95,6 | 104,5 | 112 | 119,2 | — | — |
| Х13Н13К10 | — | 90 | 100,8 | 109,6 | 115,4 | 119,6 | — | — |
| Х19Н10К47 | — | 90,5 | 98,6 | 105,2 | 110,8 | — | — | — |
| P18 | 41,9 | 47,2 | 62,7 | 81,5 | 103,7 | 117,3 | 123,6 | 128,1 |
| EH12 | 31 | 36 | 53 | 75 | 97 | 119 | — | — |
| 40Х10С2М (EI107) | 86 | 91 | 101 | 112 | 122 | — | — | — |
Chromium stainless steels
Chromium stainless steels have a high concentration of chromium atoms, which increases their resistivity - the electrical conductivity of such stainless steel is not high. At normal temperatures, its resistance is (50...60)·10-8 Ohm·m.
Electrical resistivity of chromium stainless steels ρe·108, Ohm·mSteel grade 2010030050070090011001300
| X13 | 50,6 | 58,4 | 76,9 | 93,8 | 110,3 | 115 | 119 | 125,3 |
| 2Х13 | 58,8 | 65,3 | 80 | 95,2 | 110,2 | — | — | — |
| 3Х13 | 52,2 | 59,5 | 76,9 | 93,5 | 109,9 | 114,6 | 120,9 | 125 |
| 4Х13 | 59,1 | 64,6 | 78,8 | 94 | 108 | — | — | — |
Chromium-nickel austenitic steels
Chromium-nickel austenitic steels are also stainless, but due to the addition of nickel they have a resistivity almost one and a half times higher than that of chromium steels - it reaches a value of (70...90)·10-8 Ohm·m.
Electrical resistivity of chromium-nickel stainless steels ρe·108, Ohm·mSteel grade 201003005007009001100
| 12Х18Н9 | — | 74,3 | 89,1 | 100,1 | 109,4 | 114 | — |
| 12Х18Н9Т | 72,3 | 79,2 | 91,2 | 101,5 | 109,2 | — | — |
| 17Х18Н9 | 72 | 73,5 | 92,5 | 103 | 111,5 | 118,5 | — |
| Х18Н11Б | — | 84,6 | 97,6 | 107,8 | 115 | — | — |
| Х18Н9В | 71 | 77,6 | 91,6 | 102,6 | 111,1 | 117,1 | 122 |
| 4Х14НВ2М (ЭИ69) | 81,5 | 87,5 | 100 | 110 | 117,5 | — | — |
| 1Х14Н14В2М (ЭИ257) | — | 82,4 | 95,6 | 104,5 | 112 | 119,2 | — |
| 1x14N18M3T | — | 89 | 100 | 107,5 | 115 | — | — |
| 36Х18Н25С2 (ЭЯ3С) | — | 98,5 | 105,5 | 110 | 117,5 | — | — |
| Х13Н25М2В2 | — | 103 | 112,1 | 118,1 | 121 | — | — |
| Х7Н25 (ЭИ25) | — | — | 109 | 115 | 121 | 127 | — |
| Х2Н35 (ЭИ36) | 87,5 | 92,5 | 103 | 110 | 116 | 120,5 | — |
| H28 | 84,2 | 89,1 | 99,6 | 107,7 | 114,2 | 118,4 | 122,5 |
Heat-resistant and heat-resistant steels
In terms of their electrical conductive properties, heat-resistant and heat-resistant steels are close to chromium-nickel steels. The high content of chromium and nickel in these alloys does not allow them to conduct electric current, like ordinary carbon alloys with a high concentration of iron.
The significant electrical resistivity and high operating temperature of such steels make it possible to use them as working elements of electric heaters. In particular, steel 20Х23Н18 in its resistance and heat resistance in some cases can replace such a popular alloy for heaters as nichrome Х20Н80.
Specific electrical resistance of heat-resistant and heat-resistant steels ρе·108, Ohm·mTemperature, °С15Х25Т (EI439)15Х28 (ЭИ349)40Х9С2 (ЭИХ8)Х25С3Н (ЭИ261)20Х23Н18 (ЭИ 417)Х20Н35
| 0 | — | — | — | — | — | 106 |
| 20 | — | — | 75 | 80 | — | — |
| 100 | — | — | — | — | 97 | — |
| 200 | — | — | — | — | 98 | 113 |
| 400 | 102 | — | — | — | 105 | 120 |
| 600 | 113 | — | — | — | 115 | 124 |
| 800 | — | 122 | — | — | 121 | 128 |
| 900 | — | — | — | — | 123 | — |
| 1000 | — | 127 | — | — | — | 132 |
Sources:
Copper resistivity
The relatively low resistivity of copper is an important, but not the only positive factor.
The widespread use of this material is explained by its reasonable cost and resistance to adverse external influences.
It is easy to create high-quality products of the required shape from it, which, without additional protection, retain functionality during long-term use in difficult conditions.
Various types of cable products are made from copper
Copper is the main material for conductors
The qualified selection of a suitable material is accompanied by a comprehensive assessment of several factors. The copper conductor is not damaged by corrosion because a protective layer of oxides forms on the surface.
Structural integrity is maintained at tight turning radii after repeated bending. The noted parameters are useful for equipping rooms with high humidity and laying lines of complex configurations.
However, the main advantage is the low resistance of copper wires. In addition to improving current conductivity while reducing losses during energy transmission, it should be noted that the weight and size of cable products is reduced compared to alternative options.
Resistivity of pure metals at low temperatures
Calculation of voltage drop in a cable
Vibrational processes in the molecular lattice prevent the free movement of electrons. This explains the increase in resistance as temperature increases.
A linear dependence is observed from a slight positive temperature, up to the melting point. The corresponding phase transition is accompanied by a sharp increase in electrical resistance.
Of course, such a regime after destruction is not working.
Sodium resistivity
Theoretical indicators “a” are confirmed by the results of experiment “b”. If the structure of a pure metal is distorted by impurities (impurities, alloy components), a random distribution of electric charge carriers will occur. This, in turn, will increase the circuit losses (resistance).
Metal resistance table
To be convinced of the benefits of copper, it is necessary to make an appropriate comparative analysis. Below are the metal resistance values in the summary table.
What is electrical resistance
Basic electrical parameters of conductors made from different materials
Material Resistivity in Ohms per meter, measured at room temperature (+20°C) Electrical conductivity under similar conditions, in Siemens per meter
| Copper | 1.68x10-3 | 5.96x107 |
| Silver | 1.59x10-3 | 6.3x107 |
| Gold | 2.44x10-3 | 4.1x107 |
| Aluminum | 2.82x10-3 | 3.5x107 |
| Tungsten | 5.6x10-3 | 1.79x107 |
| Iron | 1x10-7 | 1x107 |
| Platinum | 1.06x10-7 | 9.43x106 |
| Lithium | 9.28x10-8 | 1.08x107 |
Important! The low resistance of an iron conductor is not enough for the widespread use of corresponding products in practice. Active oxidation provokes rapid destruction.
Conductor resistivity table
In some situations, expenses are not considered. Military and space technology is created using conductors made of precious metals. Such solutions help reduce cross-section and weight, increase resistance to radiation and other special effects.
For the manufacture of serial products for household and industrial purposes, more affordable materials are used.
Data for calculating the electrical parameters of conductors taking into account temperature changes
Material Resistivity (in ohms per mm2/m) measured at room temperature (+0°C) Temperature correction factor (TC)
| Copper | 0,0176 | 0,004 |
| Aluminum | 0,0278 | 0,0045 |
| Steel | 0,13 | 0,0063 |
| Nikelin | 0,43-0,45 | 0,0072 |
| Brass | 0,04 | 0,002 |
| Nichrome | 0,98 | 0,0003 |
| Tungsten | 0,0612 | 0,00047 |
The use of stainless steel wire helps to increase strength while optimizing cost. To improve anti-corrosion properties, special additives are used. They increase the resistance of a steel conductor by almost 10 times compared to its copper counterpart.
In any case, the specific conditions during use, as well as the purpose of the products, are of particular importance. Nickel, for example, exhibits ferromagnetic properties at extremely low temperatures below the threshold “Curie point” (-358 0°C). Silicon, which is used to make microcircuits and transistors, has special semiconductor parameters.
Comparison of conductivity of copper and aluminum
The first conclusion can be made after studying the tabular data. Aluminum's resistance is approximately 80% higher than copper. Conductivity is worse in the same proportion. But for a correct analysis it is necessary to study additionally the following facts:
- aluminum is lighter, but to obtain similar electrical parameters you will need to increase the cross-section (conductor thickness);
- copper products (multi-core cables) are not damaged by repeated bending;
- The resistivity of aluminum changes more with increasing/decreasing temperature;
- a film of oxides forms faster on its surface, so for reliability (durability) modern wiring is made of copper.
Copper and aluminum cables are connected through a steel adapter to prevent electrochemical corrosion
Application of electrical conductivity of materials
The presence of the noted properties is used not only in engineering energy networks. Good electrical conductivity allows information signals to be transmitted over long distances without distortion.
Maintaining a high amplitude reduces the requirements for amplification paths and reduces the overall cost of systems.
Minimizing losses is useful in electrolysis installations, when creating contact groups and motor windings.
Important! In all of the above examples, in addition to a general increase in efficiency, you can count on preventing overheating.
Resistance calculation
To correct temperature changes, the last column of the second table shows separate multipliers for each position. The calculation is performed using the formula RT = Rn * (1+PC*T), where the given symbols mean:
- RT – electrical resistance in Ohms at a certain temperature;
- Rn – conductor resistance at zero temperature;
- PC – correction factor;
- T – operating temperature in degrees Celsius.
Concept of electrical resistance
This term refers to the property of creating obstacles to the passage of electric current in a circuit. The relationship between physical quantities is described by the classical formula R=U/I (symbols for resistance, voltage and current, respectively). The movement of electrons occurs under the influence of an electromagnetic field, a potential difference.
Any distortion of the crystalline structure of the molecular lattice increases the resistance of metals. This reason explains the strong dependence of the parameter on the purity of the material and temperature. Thus, standards for pipe products allow the use of various alloys.
Electrical copper (grade M006) is created with a controlled amount of foreign impurities of no more than 0.1%.
The qualified use of this material is preceded by an assessment of all significant factors. In addition to the cost, they clarify:
- features of mechanical and other types of processing;
- stability of electrical parameters under certain operating conditions;
- resistance to external influences, durability.
In some situations, the significant initial investment is justified by extended service life and reliability.
What brands of electrodes are used when welding stainless steel?
Welding stainless steel is a rather labor-intensive process, which is associated with the structural features of the material. Stainless steel electrodes allow you to obtain reliable, strong, uniform welds. Designed for connections of stainless steel structures and mechanisms.
Packaging of electrodes for stainless steel welding
Properties of stainless steel
Stainless steel has a low heat conductivity coefficient. Therefore, during welding work, heating of the local area is required to form a uniform seam. To achieve the required technical characteristics, it is necessary to install high currents on the welding machine.
To prevent overheating or scale, it is necessary to make a larger gap when joining parts than in the case of welding steel workpieces. The seam absorbs significant deformation loads during the cooling process, due to which the main structural elements maintain their geometry.
A welding electrode with a specially selected composition for specific alloys avoids overheating of the main rod. That is, the resistance of the metals is approximately the same, due to which there is no overheating process.
Welding methods
Technologies in which the use of welding electrodes for stainless steel structures is acceptable:
- pulse-arc for welding structural elements less than 0.1 mm thick, requires the use of welding electrodes with a certain coating composition;
- short-arc for welding structures whose thickness is less than 3 mm;
- plasma - a universal method that allows you to weld stainless steel of any composition;
- jet arc - used for connecting large parts with a supply of melting wire.
The welder must independently select electrodes depending on the thickness of the workpieces being joined, their composition, as well as the operating characteristics of the welded structures.
Welding recommendations:
- if you overheat the metal above +500°C, then the likelihood of crystallization cracks increases;
- when the stainless steel is heated in the range of +350°C - +500°C, the part becomes embrittled, which can lead to a loss of strength properties;
- obtaining a high-quality weld is guaranteed when the workpiece is heated to +1200°C followed by cooling for 180 minutes;
- prolonged heating of stainless steel is not recommended, as it partially loses its properties;
- when layer-by-layer welding it is necessary to bring each previous layer to +100°C;
- To set two structural elements, you need to reduce the gap between them.
Welding in most cases is carried out in a protective gas atmosphere. When choosing the composition of an electrode coating, it is necessary to take into account its thickness, strength, and properties.
When forming a seam, there is no need to sharply move the electrode along the surface. Usually, as a result of improper actions, deformations, cracks or other defects can occur inside it, as well as oxides can form.
It is important to adhere to the following rules:
- penetration of tungsten or compounds based on it into the weld pool is unacceptable; for this, the arc is ignited separately;
- The seam should be protected with a jet of argon.
The importance of using specialized electrodes
It is important to use electrodes for stainless steel for the following reasons:
- at elevated temperatures, anti-corrosion properties are lost, and the composition of the coating allows them to be preserved;
- as a result of a small coefficient of expansion, internal stresses or deformations may occur inside the seams or in the structures being connected;
- Due to low thermal conductivity, it is difficult to heat the metal evenly.
The correct choice of temperature conditions completely determines how well the weld will meet the required technical characteristics. When heated, the steel is deformed and there is a high probability of intercrystalline corrosion. Special coating compositions can prevent such negative consequences.
Markings and types of electrodes
The most common electrodes for welding stainless steel have the following markings:
Other brands of electrodes for welding stainless steel parts are less popular due to their narrow application, high cost or technical parameters.
Marked electrodes for stainless steel welding
TsL-11
TsL-11 – electrodes for welding chromium-nickel stainless steel at +450°C. Advantages of welded seams:
- reluctance to crystallization corrosion processes;
- uniformity of the deposited layer;
- During the welding process, no splashes of molten metal are formed.
OZL-6
OZL-6 – electrodes used for welding stainless steel, which is intended to be operated at elevated temperatures up to +1000°C. The advantages are identical to TsL-11.
NZh-13
NZh-13 is a brand of electrodes for welding stainless steel used in the food industry, alloys based on nickel, chromium, and molybdenum. They are used to form seams that are intended to be used at ambient temperatures up to +350°C.
Other brands
There are also other markings for stainless steel electrodes, which also allow you to obtain reliable seams:
- ZIO-8 are used for joining heat-resistant stainless steels. They are produced with a standard coating composition, allowing work to be carried out on direct or alternating current.
- NII-48G have universal application; they can weld low-alloy steels. Any convenient location relative to the surface is allowed.
- OLZ-17U is used for steels that are intended to be used in chemically active environments.
- EA for welding structural elements made of high-alloy steel alloys. Recommended for use in short-arc welding.
- OK 63.30 – electrodes for welding stainless steel of any brand.
To maintain corrosion-resistant properties, you need to use a cold welding method. It prevents the formation of carbides based on chromium or iron.
Support the channel, just read our articles, and we will post useful information about metals for you! Also visit our website, where you will find a lot of information about metals, alloys and their processing.
Conductivity of stainless steel
The classical theory of electrical conductivity of metals originated at the beginning of the twentieth century. Its founder was the German physicist Karl Rikke.
He experimentally established that the passage of a charge through a metal does not involve the transfer of conductor atoms, unlike liquid electrolytes.
However, this discovery did not explain what exactly is the carrier of electrical impulses in the metal structure.
The experiments of scientists Stewart and Tolman, conducted in 1916, allowed us to answer this question.
They were able to establish that the smallest charged particles - electrons - are responsible for the transfer of electricity in metals.
This discovery formed the basis of the classical electronic theory of electrical conductivity of metals. From this moment on, a new era of research into metal conductors began.
Thanks to the results obtained, today we have the opportunity to use household appliances, production equipment, machines and many other devices.
How does the electrical conductivity of different metals differ?
The electronic theory of electrical conductivity of metals was developed in the research of Paul Drude.
He was able to discover such a property as resistance, which is observed when electric current passes through a conductor.
In the future, this will make it possible to classify different substances according to their conductivity level. From the results obtained, it is easy to understand which metal is suitable for the manufacture of a particular cable.
This is a very important point, since incorrectly selected material can cause a fire as a result of overheating from the passage of excess voltage current.
Silver metal has the highest electrical conductivity. At a temperature of +20 degrees Celsius, it is 63.3 * 104 centimeters-1.
But making wiring from silver is very expensive, since it is a rather rare metal, which is used mainly for the production of jewelry and decorative items or bullion coins.
The metal with the highest electrical conductivity among all elements of the base group is copper.
Its indicator is 57*104 centimeters-1 at a temperature of +20 degrees Celsius.
Copper is one of the most common conductors used for household and industrial purposes.
It withstands constant electrical loads well, is durable and reliable. The high melting point allows you to work for a long time in a heated state without problems.
In terms of abundance, only aluminum can compete with copper, which ranks fourth in electrical conductivity after gold.
It is used in networks with low voltage, as it has almost half the melting point of copper and is not able to withstand extreme loads.
The further distribution of places can be found by looking at the table of electrical conductivity of metals.
It is worth noting that any alloy has much lower conductivity than the pure substance.
This is due to the merging of the structural network and, as a consequence, disruption of the normal functioning of electrons.
All given indicators are the electrical conductivity of metals, which is calculated as the ratio between the current density and the magnitude of the electric field in the conductor.
Classical theory of electrical conductivity of metals
The basic principles of the theory of electrical conductivity of metals contain six points.
First: a high level of electrical conductivity is associated with the presence of a large number of free electrons.
Second: electric current arises through external influence on the metal, during which electrons move from random motion to ordered one.
Third: the strength of the current passing through a metal conductor is calculated according to Ohm's law.
Fourth: different numbers of elementary particles in the crystal lattice lead to unequal resistance of metals.
Fifth: electric current in the circuit arises instantly after the start of exposure to electrons. Sixth: as the internal temperature of the metal increases, the level of its resistance also increases.
The nature of the electrical conductivity of metals is explained by the second point of the provisions.
In a quiet state, all free electrons rotate chaotically around the nucleus.
At this moment, the metal is not able to independently reproduce electrical charges.
But as soon as you connect an external source of influence, the electrons instantly line up in a structured sequence and become carriers of electric current. With increasing temperature, the electrical conductivity of metals decreases.
This is due to the fact that the molecular bonds in the crystal lattice weaken, elementary particles begin to rotate in an even more chaotic order, so the formation of electrons in a chain becomes more complicated.
Therefore, it is necessary to take measures to prevent overheating of the conductors, as this negatively affects their performance properties. The mechanism of electrical conductivity of metals cannot be changed due to the current laws of physics.
But it is possible to neutralize negative external and internal influences that interfere with the normal course of the process.
Metals with high electrical conductivity
The electrical conductivity of alkali metals is at a high level, since their electrons are weakly attached to the nucleus and easily line up in the desired sequence.
But this group is characterized by low melting points and enormous chemical activity, which in most cases does not allow their use for the manufacture of wires.
Metals with high electrical conductivity when opened are very dangerous for humans.
Touching a bare wire will result in an electrical burn and a powerful discharge to all internal organs. This often results in instant death.
Therefore, special insulating materials are used for the safety of people.
Depending on the application, they can be solid, liquid or gaseous.
But all types are designed to do one thing—isolating electrical current within a circuit so that it cannot affect the outside world.
The electrical conductivity of metals is used in almost all areas of modern human life, so ensuring safety is a top priority.
| The manufacturing temperature of various structures made of aluminum alloys, as a rule, does not exceed 350 degrees. This is due to the properties of aluminum - with long exposure of alloys such as avial or... |
| As a result of various treatments, the properties of steel may change. Thanks to polymorphism, which contributes to changes in the crystal lattice of the material during heating or cooling, obtaining the required steel structure becomes... |
| As for the designation of stainless steel, the marking of steels of this type is carried out in alphanumeric form, similar to the marking of structural alloy steels. It is customary to mark non-standard stainless steels using letter indices of factories...... |
| Alkaline earth metals are represented by a number of elements that belong to group II of the Mendeleev periodic system. The substances received this name due to the fact that the result of their interaction with water is the formation of an alkaline environment. If we consider the physical properties... |
Metal compatibility or how to avoid galvanic corrosion?
Contact corrosion occurs when two dissimilar metals come into direct contact.
It is impossible, for example, to connect aluminum sheets with a copper rivet, since under certain conditions they form a strong galvanic couple. Different metals have different electrode potentials. In the presence of an electrolyte, one of them acts as a cathode and the other as an anode. As a result of the chemical reaction occurring between them, a corrosion process will begin in which copper (cathode) will mercilessly destroy aluminum (anode).
Almost all pairs of dissimilar metals that are in contact with each other are subject to corrosion, since even moisture from the air can act as an electrolyte and activate their electrode potential. But some couples are more vulnerable, while others are less vulnerable.
For example, aluminum has excellent contact with galvanized steel, chromium and zinc, but brass is not at all “friendly” with steel, aluminum and zinc. To find out which metals are compatible and which are not, let's look at the basics of chemistry.
In the series of electrochemical activity, metals are in the following sequence:
For example, consider a pair of aluminum and copper. Aluminum is in the row to the left of hydrogen and has an electronegative potential of -1.7V, and copper is to the right and has a positive potential of +0.4V. A large potential difference leads to the destruction of more active aluminum. Copper is stronger than all the elements in front, so if paired with any of them, it will emerge victorious. The farther apart the elements are in a row, the higher their incompatibility and the likelihood of galvanic corrosion.
Data on the compatibility of some metals are presented in the table:
Aluminum
| Copper | Cink Steel | Iron | Lead | Stainless steel | Zinc | ||||
| Aluminum | D | N | N | N | D | ABOUT | ABOUT | D | D |
| Copper | N | ABOUT | ABOUT | D | ABOUT | N | ABOUT | N | N |
| Cink Steel | D | ABOUT | ABOUT | ABOUT | D | ABOUT | D | ABOUT | D |
| Lead | ABOUT | ABOUT | ABOUT | ABOUT | D | D | D | ABOUT | D |
| Stainless steel | D | N | N | N | ABOUT | ABOUT | ABOUT | D | N |
| Zinc | D | N | N | N | D | N | D | N | D |
D
– absolutely acceptable contacts (low risk of GC);
O
– limited permissible contacts (average risk of GC);
N
– unacceptable contacts (high risk of GC).
The table below can serve as a quick reference for determining the compatibility of certain structural metals. The permissibility and impermissibility of contacts between electrochemically dissimilar metals is established by GOST 9.005-72.
Conductivity of copper and aluminum: conductivity
Electrical conductivity or electrical conductivity is the body's ability to conduct electrical current.
This concept is extremely important in electrical engineering: metals that are good conductors are used in wires, poor conductors or dielectrics are used to protect people from electricity.
The best conductor is silver, copper is in second place (it is quite a bit inferior to silver), followed by gold and aluminum.
Advantages and disadvantages of copper wires
Copper is a ductile transition metal. It has a golden-pink color and is found in nature in the form of nuggets. It has been used by people since ancient times - an entire era was named in its honor.
The table shows the electrical resistivity of steel and other metals
Today, copper wires are often used in electronic devices. Their advantages include:
- High electrical conductivity (the metal ranks second in this indicator, second only to silver). Compared to aluminum, copper is 1.7 times more efficient: with an equal cross-section, a copper cable passes more current.
- Welding, soldering and tinning can be carried out without the use of additional materials.
- The wires have good elasticity and flexibility, they can be rolled and bent without much harm.
Copper is only slightly inferior to silver
However, until recently, copper wires were inferior to aluminum ones due to several disadvantages:
- High density: at different sizes, copper wire will weigh more than aluminum;
- Price: aluminum is several times cheaper;
- Copper oxidizes in open air: however, this does not affect its operation and is easily eliminated.
What is the resistance of copper and aluminum
Aluminum is a lightweight metal that can be easily machined and cast. It has high electrical conductivity: it ranks 4th after silver, copper and gold.
Important! Despite a number of advantages (low cost, light weight, ease of processing, and others), in the long term, aluminum wires are less profitable than copper wires.
In electrical engineering, 2 terms have meaning:
- Conductivity: Responsible for the transfer of current from one point to another. The higher the conductivity of a metal, the better it transmits electricity. At +20 degrees, the conductivity of copper is 59.5 million siemens per meter (S/m), aluminum - 38 million S/m. The conductivity of a copper cable is practically independent of temperature.
- Electrical resistance: the higher this concept, the worse the substance will pass current. The resistivity of copper is 0.01724-0.0180 μOhm/m, aluminum - 0.0262-0.0295.
Aluminum cables are in demand no less than copper ones
In other words, copper has higher conductivity and lower resistance than aluminum.
What is the resistivity of steel
Steel is a metal alloy of iron with carbon and other elements. It contains at least 45% iron, the carbon content ranges from 0.02% to 2.14%. Depending on the exact composition, steel is used in construction, mechanical engineering and instrument making, as well as in many areas, for example, in transport, the national economy, and in the production of household appliances.
Steel wires have low conductivity
The conductivity of steel is only 7.7 million S/m, the resistivity is 0.13 μOhm/m, that is, it is quite high. Steel conducts electricity poorly and is not used in the production of cables.
However, you can often find an outer galvanized steel braid that protects the wires from mechanical stretching.
Such protection is needed if the cable runs under a road or on unstable ground, if there is a risk of sharply pulling the wire.
PNSV is also made from steel - a heating wire with a steel core and vinyl insulation. It is placed inside the structure before concrete is poured and is subsequently used for electrical heating of the finished block. The cable practically does not conduct electricity.
PNSV wire is produced from steel
Comparison of conductivity of different types of steel
The characteristics of steel depend on its composition and temperature:
- For carbon alloys, the resistance is quite low: it is 0.13-0.2 μOhm/m. The higher the temperature, the greater the value;
- Low-alloy alloys have a higher resistance - 0.2-0.43 μOhm/m;
- High-alloy steels have high resistance - 0.3-0.86 μOhm/m;
- Due to the high chromium content, the resistance of chromium stainless alloys is 0.5-0.6 μOhm/m;
- Chromium-nickel austenitic steels are stainless and, thanks to nickel, have a high resistance of 0.7-0.9 μOhm/m.
Galvanized braiding is often made from steel
Copper ranks second in terms of electrical conductivity: it perfectly passes electric current and is widely used in the manufacture of wires. Aluminum is also used no less often: it is weaker than copper, but cheaper and lighter.
What is electrical conductivity?
The history of aluminum-copper alloys begins with the experiments of H. Oersted in 1825, when he wanted to obtain pure Al by electrolysis. In fact, he received a certain composition, which included other elements participating in the experiment.
Further experiments on the discovery of pure aluminum were carried out by F. Wöhler in 1827, when he received 30 grams of Al powder, and in 1845 - molten balls. However, the production method was too labor-intensive and required improvement.
In 1856, A. Deville and his research group developed an industrial method for producing aluminum and opened its first mass production. In 1886, P. Heroux and C. Hall discovered the electrolytic method, which turned out to be cheaper and more effective than the chemical one.
From 1888 to 1895, factories for the mass production of Al were opened in Neuhausen (Switzerland).
In 1906, A. Wilm began to develop high-strength aluminum-copper alloys at his own enterprise. Through experiments, he obtained a sample that had the property of self-strengthening. Its production continued in 1911 in Germany.
Massive research into alloys took place from 1920 to 1940 in the USSR, Germany, and the USA. Two directions of experiments began to clearly separate - the study of pure and doped compositions.
Composition and structure
The phase diagram of Al-Cu aluminum alloys has the following features:
- The maximum solubility of copper in aluminum in the solid phase is 5.65%, which decreases with decreasing temperature. This makes hardening and aging possible. The CuAl2 phase plays a strengthening role using the solution method, imparting mechanical and thermal strength.
- The eutectic point is at 33% copper concentration and consists of a brittle but strong CuAl2 phase, which makes the material unsuitable for practical use. A large amount of copper significantly increases the density of the samples. For casting, alloys with a concentration of 1 to 1.5% (to obtain strengthening) and 6 to 8% (to eliminate the amount of brittle CuAl2 phase) are used.
- The good solubility of Cu in Al and the low melting point of the eutectic +5480C cause the appearance of a wide crystallization range.
Low fluidity, the formation of pores, cracks, and segregation are characteristic signs of the need to find a compromise between casting and strength properties.
The main alloying element is copper, which leads to the creation of a nonequilibrium eutectic phase. Therefore, during heat treatment by quenching, the melt is heated stepwise to +5300C, followed by holding until a stable phase is obtained.
A significant number of conduction electrons in Cu-Al alloys significantly reduce the electrical resistivity to a level of less than 0.02 μOhm*m. The presence of iron impurities or alloying elements has virtually no effect on this value.
Aluminum
Useful tips
In conclusion, here are a few tips that should be taken into account when organizing wiring:
- In case of independent design of wiring in a house or apartment, it is better to choose copper wires. With a smaller cross-section, they can withstand greater currents and are more resistant to frequent bending. An equally important point is volume. Copper wires are compact, which simplifies the process of creating grooves. For example, when connecting a receiver with a power of 7-8 kW, the aluminum wire should have a cross-section of about 8 mm. The cable has three cores plus a braid. As a result, the total diameter is about 1.5 centimeters. For comparison, copper can have a cross-section of 4 sq. mm, and the overall diameter is no more than a centimeter.
- When installing a socket, a three-core cable with a ground wire must be used. The distance of the socket from the floor is 30 cm. When organizing a lighting circuit, it is allowed to use cables with two cores (grounding is not needed here).
- It is forbidden to hang the entire load on one pair of wires (especially if they are aluminum). The best option is to divide the circuit into several lines. For example, a bathroom is powered through one machine, lighting through another, a kitchen through a third, and so on. The wire cross-section for the kitchen and bathroom should be 4 or 6 sq. mm, and for the lighting circuit - 1.5 or 2.5 mm.
Read also: Artistic forging railings for stairs photo
The most difficult situation is in old apartments where aluminum wires are installed, which have outlived their useful life and require replacement. Wiring with a cross-section of 2.5 square meters.
mm can withstand a load of no more than 20 Amps, which is not enough for modern electrical receivers. In addition, the insulation of the wires loses its elasticity over time and gradually deteriorates.
In such a situation, the only solution is to completely replace the wiring with copper wires.
For more information on why it is worth replacing aluminum wiring with copper in an old house, watch this video: