Today people are extremely dependent on fuel. Heating of homes, cooking, operation of equipment and vehicles cannot be done without it. Most of the fuels used are hydrocarbons. To assess their efficiency, specific heat of combustion values are used. Kerosene has a relatively impressive indicator. Due to this quality, it is used in rocket and aircraft engines.
Due to its properties, kerosene is used in rocket engines
What is this?
Kerosene is a flammable mixture consisting of liquid hydrocarbons.
The boiling point of kerosene varies between 150-250° Celsius. It is a clear, colorless (in some cases yellowish) liquid, slightly oily to the touch. The word itself comes from English. kerosene. In turn, it has Greek roots: κηρός - “wax”.
Kerosene is obtained by direct distillation or ratification of petroleum. Sometimes - through its recycling. In some cases, the product is hydrotreated.
Kerosene as fuel:
Kerosene (English kerosene from ancient Greek κηρός - “wax”) is a flammable mixture of liquid hydrocarbons (from C8 to C15) with a boiling point in the range of 150-250 °C, obtained by direct distillation or rectification of oil.
Externally, kerosene is a transparent, colorless (or slightly yellowish, or light brown), slightly oily liquid to the touch. Has a characteristic smell of petroleum products.
Kerosene is a flammable, flammable liquid. Refers to low-hazard substances and, in terms of the degree of impact on the human body, in accordance with GOST 12.1.007, belongs to the 4th hazard class. Combustible fuel.
Kerosene is lighter than water. Does not dissolve in water.
Kerosene forms explosive mixtures with air
Composition of the substance
We figured out the boiling point of kerosene. Now let's imagine the composition of this product. It is not universal and standard, since it depends on the raw material - oil, its processing method and chemical composition.
So, the composition of kerosene according to GOST:
- Aliphatic saturated hydrocarbons - 20-60% of the total mass.
- Naphthenic hydrocarbons - 20-50%.
- Aromatic bicyclic hydrocarbons - 5-25%.
- Unsaturated hydrocarbons - up to 2%.
- Insignificant content of impurities - sulfur, oxygen or nitrogen.
Let us now imagine the most important properties of this substance.
Basic thermophysical characteristics of kerosene
Kerosene is the middle distillate of the petroleum refining process, defined as the fraction of crude oil that boils at a temperature between 145 and 300°C. Kerosene can be produced by distilling crude oil (straight-run kerosene) or from cracking heavier petroleum streams (cracking kerosene).
Crude kerosene has properties that make it suitable for blending with a variety of performance additives for use in a variety of commercial applications, including transportation fuels. Kerosene is a complex mixture of branched and straight chain compounds that can generally be divided into three classes: paraffins (55.2% by weight), naphthenes (40.9%) and aromatics (3.9%).
To be effective, all grades of kerosene must have the highest possible specific heat of combustion and specific heat capacity, and also be characterized by a fairly wide range of ignition temperatures. For different groups of kerosenes these indicators are:
- Specific heat of combustion, kJ/kg - 43000±1000.
- Self-ignition temperature, 0C, not lower than – 215.
- Specific heat capacity of kerosene at room temperature, J/kg K – 2000…2020.
It is impossible to accurately determine most of the thermophysical parameters of kerosene, since the product itself does not have a constant chemical composition and is determined by the characteristics of the original oil. In addition, the density and viscosity of kerosene depends on external temperatures. It is only known that as the temperature approaches the zone of stable combustion of the petroleum product, the specific heat capacity of kerosene increases significantly: at 2000C it is already 2900 J/kg K, and at 2700C it is 3260 J/kg K. Accordingly, the kinematic viscosity decreases. The combination of these parameters determines good and stable ignition of kerosene.
Temperature of combustion of kerosene in air
Kerosene
is a mixture of hydrocarbons with an atomic number of more than 9 and less than 16, which boil away during the direct distillation of oil in the temperature range of + 100, + 320 degrees Celsius.
Chemical composition and properties of kerosene
The chemical composition of kerosene obtained by cracking may vary depending on the type of oil it is derived from, as well as the technology used for its processing and further purification of the kerosene distillate. On average, this petroleum product may include:
- alifactic carbons in percentage from 20 to 60;
- naphthenic carbons in percentage from 20 to 50;
- bicyclic aromatic carbons in percentage from 5 to 25;
- unsaturated carbons in percentage up to 2.
At higher temperatures of kerosene
the amount of bicyclic aromatic carbons increases.
At the same time, their lower content in the finished petroleum product helps to increase the intensity and brightness of the flame.
A high percentage of heavy fractions leads to deterioration in the combustion of this petroleum product, therefore, after its production, special chemical and hydrotreating is carried out.
The high volatility of this product should also be taken into account. When the concentration in the air exceeds 300 mg/m3, there is a risk of poisoning by kerosene vapors. This imposes certain requirements on the storage conditions of this petroleum product.
Kinematic viscosity
When characterizing kerosene according to GOST, this position will also be relevant. It must be said that the viscosity of the hydrocarbons included in this product changes significantly with a decrease/increase in its temperature. The higher the latter is, the lower the viscosity becomes.
This is a very important characteristic. The viscosity of kerosene has a great influence on a number of operational features of aircraft fuel systems, as well as combustion and mixture formation processes in the engine.
Thus, the viscosity of kerosene at 20 °C is 1.2 - 4.5 mm2/s.
How to make 80 from 92 gasoline
Using the first formula, we find out the amount of kerosene per liter of the resulting 80th:
K= (92-80) / (92-45) = 12 / 47 ≈ 0.255 l
Using the third formula, we calculate how much kerosene needs to be added to 10 liters of 92 gasoline:
Kk= (10 ⋅ 0.255) / (1-0.255) = 2.55/0.745 ≈ 3.42 l
Bottom line: to get 80-grade gasoline from 10 liters of 92-grade gasoline, you need to pour 3.42 liters of kerosene into the second one.
Very often I am asked what we fueled the engines of Victory during the media expedition in 2022. Indeed, if in the vastness of our homeland it was still possible to find the 80th, then in Europe, especially in the eurozone, there is not even a 92nd, the 95th is rarely found, mainly the 98th, 100 and 102nd. Let me remind you that the Pobeda M20 engine has a compression ratio of 6.2 and is designed to run on 66-octane gasoline. We discussed for a long time how to solve this problem before the trip; there were options to boost the engines, order new camshafts with valve timing for high-octane fuel, change the valves to valves with soda, reduce the compression ratio by reducing the combustion chamber, etc. All this turned out to be labor-intensive and time-consuming, and also quite costly in terms of finances. In the end, it was decided to use a special fuel additive. So, during the trip in our Victories (all 4 cars) we used a fuel additive, the so-called tetraethyl lead. The photo shows a bottle with it.
The difference between kerosene and gasoline
The method for producing aviation kerosene is direct distillation of low-sulfur and sulfur oil. To improve the physicochemical properties of kerosene, various additives and hydrotreating are used. Kerosene has a number of advantages over gasoline:
- high heat of combustion (both mass and volumetric);
- low volatility;
- lower freezing point;
- low kinematic viscosity.
- In addition, kerosene is less fire hazardous than gasoline.
A significant advantage in using kerosene is its breadth of application. In addition to fuel for jet power plants, it is used on board as a coolant or coolant for radiators. To control the cross-section of the engine nozzle, a hydraulic system is used, the working fluid of which can also be kerosene. Needless to say, this type of fuel is an excellent solvent. This is extremely important when organizing the maintenance process of jet aircraft engines.
Kerosene
Kerosene for lighting: its physical and chemical properties are close to gasoline. Density 0.7940 (20°C); The content of aromatic hydrocarbons is 19.4%, alicyclic 39.2%, methane 41.4% (during the processing of oil from the Kalamkass field, it is released in small quantities). [thirty]
Diesel fuel is heavier than gasoline and kerosene. Contains a significant amount of hydroaromatic (naphthenic) structures, as well as paraffin, normal and branched hydrocarbons. Average density at 20°C is 0.800 g/cm 3 (0.83 – DL Kal amkassa). Content of paraffin hydrocarbons: n – structure 36.2 wt%, structure 17.2 wt%. Naphthenic –
23 wt%, aromatic – 21.9 wt%, olefinic – 1.7 wt%.[30]
High-viscosity residues from the primary distillation of oil and a residue similar in properties to fuel oil after distillation of visbreaking products. They are a mixture of solid and liquid hydrocarbons, their sulfur-, oxygen-, and nitrogen-containing derivatives. Density more than 0.9 g/cm3. They contain a large amount of asphalt-resinous substances - 42.1% in the fuel oil of the primary distillation of oil from the Kalamkass field. Content of paraffin hydrocarbons: n - 19.7 wt%, and - 6.0 wt%, aromatic - 39.5 wt%. [1] '
Cyclic hydrocarbon of the hydronaphthalene series. Molecular
mass 138.25 kg/kmol. Density 900 kg/m. Well soluble in petroleum products. [2]
Solid combustible substance, density 1960 – 2070 kg/m3. Boiling point 446.6°C, melting point 112.8 – 119.3°C.[25]
1.2 TOXIC PROPERTIES
Oils containing few aromatic hydrocarbons also act as mixtures of methane and naphthenic hydrocarbons; their vapors cause anesthesia and convulsions. A high content of aromatic hydrocarbons can threaten chronic poisoning with changes in the composition of the blood and hematopoietic organs. Sulfur compounds in oil can cause acute and chronic poisoning; Hydrogen sulfide plays a major role in this. Instant poisoning by volatile compounds of sulfurous oil is described at a concentration of hydrogen sulfide of 0.55 - 0.63 mg/l and hydrocarbons of 15-20 mg/l. Oil causes acute poisoning, congestion of the brain, pulmonary edema, possible hemorrhages, impaired sense of smell, and excitability of the nervous system, headache, weakness, palpitations, pain in the heart area. Causes skin irritation and skin lesions, both acute and chronic. [thirty]
Density
One of the most important characteristics used for all petroleum products. And if we compare the density of kerosene and water, we will see that the latter will be higher. Here are the specific numbers:
- The density of distilled water at an “ideal” temperature of 3.7 °C is 1000 kg/m3.
- The density of sea water at an “ideal” temperature of 3.7 °C is 1030 kg/m3.
- The density of boiling water at 100 °C is 958.4 kg/m3.
To further compare the density of water and kerosene, let’s get acquainted with this characteristic regarding the petroleum product. This is 800 kg/m3.
It must be said that in the early stages of the development of the oil industry, density was the only characteristic of kerosene. Today, in practice, the quantity most often used is relative density. This is a dimensionless indicator equal to the ratio of the true densities of a given petroleum product and distilled water, taken for comparison at certain temperatures.
Thus, the density of kerosene at 20 °C will be from 780 to 850 kg/m3.
Compound
Their composition depends on the composition of the burning substance and its combustion conditions. In fire conditions, organic substances most often burn (wood, fabrics, gasoline, kerosene, rubber, etc.), which consist mainly of carbon, hydrogen, oxygen and nitrogen. When they burn in a sufficient amount of air and at high temperatures, the products of complete combustion are formed: CO2, H2O, N2. When burning in an insufficient amount of air or at a low temperature, in addition to the products of complete combustion, incomplete combustion products are formed: CO, C (soot).
Combustion products are called wet
, if the content of water vapor is taken into account when calculating their composition, and
dry
, if the content of water vapor is not included in the calculation formulas.
Less commonly, inorganic substances burn during a fire, such as sulfur, phosphorus, sodium, potassium, calcium, aluminum, titanium, magnesium, etc. Their combustion products in most cases are solid substances, for example P2O5, Na2O2, CaO, MgO. They are formed in a dispersed state, so they rise into the air in the form of dense smoke. The combustion products of aluminum, titanium and other metals are in a molten state during the combustion process.
With incomplete combustion of organic substances under conditions of low temperatures and lack of air, more diverse products are formed - carbon monoxide, alcohols, ketones, aldehydes, acids and other complex chemical compounds. They are obtained by partial oxidation of both the fuel itself and the products of its dry distillation (pyrolysis). These products produce acrid and poisonous smoke. In addition, the products of incomplete combustion themselves are capable of burning and forming explosive mixtures with air. Such explosions occur when extinguishing fires in basements, dryers and in enclosed spaces with a large amount of flammable material. Let us briefly consider the properties of the main combustion products.
Carbon dioxide
Carbon dioxide or carbon dioxide (CO2) is the product of complete combustion of carbon. It is odorless and colorless. Its density relative to air is 1.52. The density of carbon dioxide at temperature T = 0 °C and at normal pressure p = 760 millimeters of mercury (mm Hg) is equal to 1.96 kg/m3 (the density of air under the same conditions is ρ = 1.29 kg/m3). Carbon dioxide is highly soluble in water (at T = 15 °C, one liter of gas dissolves in one liter of water). Carbon dioxide does not support combustion of substances, with the exception of alkali and alkaline earth metals. The combustion of magnesium, for example, occurs in an atmosphere of carbon dioxide according to the equation:
CO2 +2 Mg = C + 2 MgO.
The toxicity of carbon dioxide is negligible. A concentration of carbon dioxide in the air of 1.5% is harmless to humans for a long time. When the concentration of carbon dioxide in the air exceeds 3-4.5%, staying indoors and inhaling the gas for half an hour is life-threatening. At temperature T = 0 °C and pressure p = 3.6 MPa, carbon dioxide turns into a liquid state. The boiling point of liquid carbon dioxide is T = –78 °C. With the rapid evaporation of liquid carbon dioxide, the gas cools and turns into a solid state. Both in liquid and solid states, drops and powders of carbon dioxide are used to extinguish fires.
Carbon monoxide
Carbon monoxide or carbon monoxide (CO) is a product of incomplete combustion of carbon. This gas is odorless and colorless, making it particularly dangerous. The relative density is 0.97. The density of carbon monoxide at T = 0 °C and p = 760 mm Hg is 1.25 kg/m3. This gas is lighter than air and accumulates in the upper part of the room during fires. Carbon monoxide is almost insoluble in water. Capable of burning and forming explosive mixtures with air. Carbon monoxide produces a blue flame when burned. Carbon monoxide is very toxic. Inhaling air with a carbon monoxide concentration of 0.4% is fatal to humans. Standard gas masks do not protect against carbon monoxide, so in case of fires, special filters or oxygen isolating devices are used.
Water
Well-known water - H2O - is also released during combustion in the form of gas - like steam. Water is a product of combustion of methane gas – CH4. In general, water and carbon dioxide are mainly released during the complete combustion of all organic substances.
Hydrogen cyanide
Potassium cyanide is a powerful poison - a salt of hydrocyanic acid, also known as hydrogen cyanide - HCN. It is a colorless liquid, but very volatile (easily turning into a gaseous state). That is, during combustion it will also be released into the atmosphere in the form of gas. Hydrocyanic acid is very poisonous, even a small - 0.01 percent - concentration in the air is fatal. A distinctive feature of the acid is the characteristic smell of bitter almonds. But hydrocyanic acid has one “zest” - it can be poisoned not only by inhaling directly through the respiratory system, but also through the skin. So you won’t be able to protect yourself only with personal respiratory and vision protection.
Acrolein
Propenal, acrolein, acrylic acid are all names for one substance, the unsaturated aldehyde of acrylic acid: CH2 = CH-CHO. This aldehyde is also a highly volatile liquid. Acrolein is colorless, has a pungent odor, and is very poisonous. If liquid or its vapor comes into contact with mucous membranes, especially the eyes, it causes severe irritation. Propenal is a highly reactive compound, which explains its high toxicity.
Formaldehyde
Like acrolein, formaldehyde belongs to the class of aldehydes and is an aldehyde of formic acid. This compound is also known as methanal. It is a toxic, colorless gas with a pungent odor.
Nitrogen-containing substances
Most often, during the combustion of substances containing nitrogen, pure nitrogen is released - N2. This gas is already contained in large quantities in the atmosphere. Nitrogen can be an example of a combustion product of amines. But during thermal decomposition, for example, of ammonium salts, and in some cases during combustion itself, its oxides are also released into the atmosphere, with the oxidation degree of nitrogen in them plus one, two, three, four, five. Oxides are gases that are brown in color and extremely toxic.
Sulphur dioxide
Sulfur dioxide (SO2) is a product of combustion of sulfur and sulfur compounds. Colorless gas with a characteristic pungent odor. The relative density of sulfur dioxide is 2.25. The density of this gas at T = 0 °C and p = 760 mm Hg is 2.9 kg/m3, that is, it is much heavier than air. Sulfur dioxide dissolves well in water, for example, at a temperature T = 0 °C, eighty liters of SO2 dissolve in one liter of water, and forty liters at T = 20 °C. Sulfur dioxide does not support combustion. It irritates the mucous membranes of the respiratory tract and is therefore very toxic.
Smoke
When many substances burn, in addition to the combustion products discussed above, smoke - a dispersed system consisting of tiny solid particles suspended in a gas. The diameter of smoke particles ranges from 10−4 to 10−6 cm (1 to 0.01 μm). Note that 1 micron (micron) is equal to 10−6 m or 10−4 cm. Larger solid particles formed during combustion quickly settle in the form of soot and soot. When organic matter burns, the smoke contains solid soot particles suspended in CO2, CO, N2, SO2 and other gases. Depending on the composition and combustion conditions of the substance, fumes of different composition and color are obtained. When wood burns, for example, it produces grayish-black smoke, fabric produces brown smoke, oil products produce black smoke, phosphorus produces white smoke, paper, straw produces whitish-yellow smoke.
The smoke generated in fires during the combustion of organic substances, in addition to the products of complete and incomplete combustion, contains products of thermal-oxidative decomposition of combustible substances. They are formed when heating flammable substances that are not yet burning and are in an environment of air or smoke containing oxygen. This usually occurs in front of the flame or in the upper parts of rooms where heated combustion products are located.
On this topic ▼
Combustible substances and materials
Types, groups, requirements, storage order
The composition of the products of thermal oxidative decomposition depends on the nature of the combustible substances, temperature and conditions of contact with the oxidizer. Thus, studies show that during the thermal-oxidative decomposition of flammable substances, the molecules of which contain hydroxyl groups, water is always formed. If flammable substances contain carbon, hydrogen and oxygen, the products of thermal oxidative decomposition most often are hydrocarbons, alcohols, aldehydes, ketones and organic acids. If the composition of flammable substances, in addition to the listed elements, contains chlorine or nitrogen, then the smoke also contains hydrogen chloride and cyanide, nitrogen oxides and other compounds. Thus, the smoke when burning nylon contains hydrogen cyanide, when burning Relin linoleum - hydrogen sulfide, sulfur dioxide, when burning organic glass - nitrogen oxides. The products of incomplete combustion and thermal-oxidative decomposition in most cases are toxic substances, therefore extinguishing fires in premises is carried out only in oxygen insulating gas masks.
Ash, ash, soot, soot, coal
Soot, or soot , is the remains of carbon that has not reacted for various reasons. Soot is also called amphoteric carbon. Ash, or ashes , are small particles of inorganic salts that have not burned or decomposed at combustion temperatures. When fuel burns out, these microcompounds become suspended or accumulate at the bottom. And coal is a product of incomplete combustion of wood, that is, its remains that have not burned, but are still capable of burning. Of course, these are not all the compounds that will be released during the combustion of certain substances. It is unrealistic to list them all, and it is not necessary, because other substances are released in negligible quantities, and only during the oxidation of certain compounds.
Flash point
The next characteristic after the boiling point of kerosene is the flash point. This is a parameter that determines the degree of fire hazard of a given liquid. Here the flash point of kerosene will vary from 28 to 60 °C.
It must be said that this characteristic is strictly controlled by standards to prevent gasoline from entering the fuel, which can dramatically increase its flammability. The practical determination of the temperature of reactive flashes of kerosene liquid is prescribed by the standards of all countries of the world.
Auto-ignition temperature
Next up is another thermal indicator - the ignition temperature of kerosene. This characteristic should be understood as the ignition of the steam-air mixture, which leads to combustion. However, ignition of vapors will not always be a sufficient condition for stable combustion of kerosene.
The auto-ignition temperature is the lowest temperature at which petroleum product vapors together with air can ignite without the presence of an ignition source. By the way, the functioning of diesel internal combustion engines is based on this remarkable property.
Self-ignition of kerosene will occur at a temperature of 300 °C.
Petrol
The fuel is most popular, especially among car owners. It consists of a mixture of hydrocarbons, nitrogen, sulfur, and oxygen. There are different brands of gasoline. Each of them contains more or less of the listed components. Because of this, performance characteristics differ.
Evaporation temperature
The term refers to the thermal threshold, after passing which gasoline spontaneously mixes with air. It cannot be determined using one number.
This value depends on the following factors:
- saturated vapor pressure;
- fractional composition;
- tension surface viscosity;
- density;
- heat capacity.
The evaporation temperature of gasoline of different compositions does not differ too much from each other. This occurs at 30°C, and if the fractions are heavy – 205°C. When it's cold outside, gasoline needs to expend more energy to get into the combustion chamber and start the engine.
Boiling temperature
Young car enthusiasts do not know that in the heat, when the fuel boils in the carburetor, the car could become immobilized. There were plugs in the fuel system due to overheating of the light fractions. They separated from the heavy ones, becoming gas bubbles. The vehicle needed to cool down and then continue traveling.
Flash point
The oil product does not have its own formula. It includes many components. Gasoline can ignite at -40 °C if an open fire occurs.
Combustion temperature
The octane number does not affect it. Only resistance to detonation depends on it. Popular brands of gasoline have almost the same characteristics. The temperature in the engine is 900-1100 °C, maybe lower. This is affected by cylinder pressure. As for open fire, for gasoline it is 800-900 °C.
What is aviation fuel?
Fuel for use in the aviation industry is a flammable substance intended to be supplied mixed with air into the combustion chamber of an aircraft engine. The goal is to obtain thermal energy, which is released at the moment of oxidation of the mixture with oxygen, that is, combustion. The fuel poured into the coffered tanks of aircraft is divided into two types.
Aviation gasoline
This type of fuel is obtained using direct distillation, reforming or catalytic cracking. The main physical and chemical indicators of aviation gasoline are:
- resistance to detonation;
- chemical stability;
- factional composition.
Gasoline is characterized by high volatility and suitability for the formation of fuel-air mixtures necessary for current flight conditions.
This type of combustible mixture is used for combustion in piston internal combustion engines. Airplanes with such engines fly short distances on local airlines and are used for demonstration flights and air shows. The most popular brands in Russian small aviation were the brands of leaded gasoline for normal and lean mixtures, developed in the last quarter of the last century - B91/115 and B95/130. Today, the small aircraft fleet is fully fueled with regular AI-95 gasoline or imported AVGAS 100LL fuel.
Interesting: Why do children suck their thumb? Reasons, what to do, photos and videos
Aviation kerosene
Regular gasoline is not suitable for combustion in the combustion chamber of a turbojet aircraft engine . Piston engines use the effect of sudden ignition of the gasoline-air mixture to create a shock at the cylinder head. A completely different principle is used in jet engines. It is important here that the combustion is smooth. This is exactly what burnt aviation kerosene provides.
To fill the caissons of jet aircraft, fuel is used, which is obtained from the middle distillate kerosene fraction of oil with a boiling point of 150-280°C. 96-98% of the composition of aviation kerosene is naphthenic, paraffin and aromatic hydrocarbons. The rest of the composition comes from resins, nitrogenous and organometallic compounds.
Effect on the human body
The degree of toxicity of substances is related to their physical and chemical nature. Interacting with the body, combustion products cause pathological syndromes.
The International Classification of Diseases, Tenth Revision ICD-10, defines poisoning by combustion products with code T59 - “Toxic effects of other gases, fumes and vapors.”
According to the mechanism of action on humans, toxic components in smoke are divided into five groups.
On this topic ▼
Carbon monoxide poisoning
Symptoms, first aid and prevention
- Substances that cause damage to the skin and mucous membrane. Symptoms of such poisoning by combustion products are itching, burning of the skin and its inflammation, pain in the eyes, eyelids, lacrimation, cough. Examples are tar fumes, sulfur dioxide, formaldehyde.
- Combustion products that cause acute inhalation poisoning. The victims complain of shortness of breath and cough. Upon examination, rapid breathing and cyanosis are noteworthy. High concentrations of toxic gas may cause respiratory arrest. Thus, signs of poisoning by PVC combustion products may appear within a few hours. Inhalation poisoning is caused by chlorine, ammonia, and nitric oxide.
- Combustion products produce toxic substances, which are called “blood poisons.” By binding hemoglobin, they disrupt the access of oxygen to tissues and trigger pathological reactions that affect the entire body. Examples: carbon monoxide, nitrogen dioxide.
- Combustion products for which the target organ is the nervous system. This is benzene, hydrogen sulfide.
- Enzyme poisons that affect tissue respiration, blocking oxygen activation processes. This is hydrogen sulfide, hydrocyanic acid.
Many toxins that form in combustion products are “universal”, since they cause damage to several body systems at once.
GNP
This abbreviation refers to the height of the non-smoking flame of the petroleum product. In particular, this is an important characteristic for KO-25 kerosene. Determines its ability to burn in a standard wick lamp (with a wick diameter of 6 mm) with a white, uniform flame without the formation of soot or soot.
This is a numerical indicator measured in millimeters. It must be indicated on the labels of the corresponding lighting brands of the product. GNP is directly influenced by the chemical and fractional compositions of kerosene.
Combustion of fuelsHydrocarbon fuels are characterized by high combustion speed and completeness.
Thanks to this, the engine receives a high-density thermal charge for its operation in a very short period of time. With a well-organized process, the completeness of combustion of hydrocarbon fuels reaches 98% or more. The amount of heat released during the combustion of hydrocarbon fuels is much greater than during the combustion of an equal amount of such “classical” fuel as coal.
Due to the rapidity of the process, organizing the combustion of hydrocarbon fuels with better selection and use of the generated heat is a difficult task.
The study of the theory and practice of combustion of various fuels has made it possible to achieve significant success. Some combustion patterns have been clarified and subjected to mathematical processing. However, to date it is still practically impossible to completely simulate or break down into simpler components such a complex phenomenon as the combustion of fuels.
The process of combustion of hydrocarbons is their oxidation by atmospheric oxygen, which occurs at a very high speed, to the final reaction products - mainly water and carbon dioxide. Among the products of incomplete combustion there may be very small amounts of CO, H2, CH4 and solid particles consisting almost entirely of carbon. The combustion temperature of hydrocarbon-air mixtures exceeds 2000 °C.
The initial ignition of the working mixture occurs due to an external source (electric spark, hot engine parts). The source temperature can reach 1000 °C.
A distinction is made between diffusion-chain and thermal propagation of flame under stationary combustion conditions. In the combustion zone, where the temperature reaches several thousand degrees, the main role is played by the processes of heat transfer from the combustion zone to a fresh portion of the working mixture by thermal conductivity and diffusion. Since this mechanism of flame propagation is predominantly thermal in nature, the main factor accelerating the reaction is temperature.
The beginning of combustion of injected and atomized fuel is preceded by its evaporation from the surface of the droplets. Only after this the resulting fuel vapors mix with air and burn. In this way, homogeneous gas mixtures are burned.
As in any chemical reaction, the combustion rate is determined by the change in time of the concentration of the reacting substances or the resulting reaction products. It depends on the chemical and physical properties of the reacting substances, as well as on the reaction conditions (heat of combustion, heat of vaporization, specific heat of fuel vapor, temperature, volume and surface of the reaction space, pressure, etc.).
Radicals are the primary active particles that cause the development of branched chain reactions in the combustion zone. They easily combine with oxygen, as well as with starting or intermediate products, without any additional activation. Self-heating of the reacting mixture leads to progressive self-acceleration of the combustion reaction. Self-ignition and ignition of the working mixture are characterized by a transition from the relatively slow development of pre-flame oxidation reactions to a very fast chemical process, accompanied by the appearance of a flame and combustion of hydrocarbons almost completely to the final products of their oxidation.
In compression ignition engines (diesels), self-heating and ignition of the working mixture occur due to an imbalance between the rate of heat release during oxidation reactions and heat removal from the reacting system to the environment. Ignition that occurs due to an imbalance in heat is sometimes called a thermal explosion or thermal autoignition. For diesel fuels, the self-ignition of which is thermal in nature, the most important characteristic is the duration of the ignition delay or flammability (cetane numbers).
Ignition from a heated surface or an extraneous source (spark) has much in common with chain thermal self-ignition in a volume. It also occurs as a result of progressive self-acceleration of pre-flame reactions, although reactions develop mainly in the hottest layers near the hot surface. In this case, the nature of the ignition is point-like, and not a shock wave.
The maximum values of normal flame propagation speed used in engines with hydrocarbon fuels mixed with air range from 35-55 cm/sec at atmospheric pressure and temperature 20 °C. The lowest combustion rates under comparable conditions are characterized by alkanes, the highest by diolefins and acetylene hydrocarbons. Cyclans and aromatic hydrocarbons occupy an intermediate position. With an increase in the number of carbon atoms in the molecule, this difference is leveled out to a certain extent.
Burning and propagation of laminar flame flow for a hydrocarbon-air mixture is possible only within the following known concentration limits (at atmospheric pressure and temperature 20 °C):
The rate of fuel combustion increases greatly if the combustible mixture is in intense vortex (turbulent) motion. Accordingly, the intensity of turbulent heat transfer can be significantly higher than with molecular diffusion.
The speed of propagation of a turbulent flame increases in direct proportion to the increase in the rate of hydrocarbon oxidation reaction, and, consequently, to the combustion temperature. Numerous attempts are being made to influence the combustion rate by selecting fuel of a certain chemical composition, as well as by introducing combustion-initiating additives.
In the case of organizing the combustion process by atomizing fuel using nozzles, the combustion rate will be determined to a large extent by the rates of evaporation of droplets and diffusion mixing of the resulting fuel vapors with air.
So, the speed and completeness of combustion is influenced by many factors, of which very important ones are: the chemical nature of the fuel, the uniformity of composition and distribution of the working mixture (fuel-air) in the combustion chamber. For internal combustion engines with compression ignition, the act of self-ignition of liquid atomized fuels in the cylinder is no less important. There is always a known time gap between the start of diesel fuel injection and the start of its combustion, which is considered as a delay in self-ignition, characterizing the quality of the fuel in terms of flammability, and therefore the start and combustion process.
The combustion rate of atomized liquid fuel in an engine depends not only on the chemical oxidation reaction, but also on purely physical processes, which primarily include evaporation and diffusion. Frank-Kamenetsky believes that in the heterogeneous combustion process of liquid atomized fuel, two limiting regions can always be distinguished: kinetic and diffusion. In areas of high temperatures, where the rate of chemical reaction is extremely high, the total rate of the heterogeneous reaction will be determined only by the rate of diffusion of fuel vapor and air oxygen. Diffusion is complicated by convection currents caused by temperature differences in different parts of the combustion chamber and vortex air movements.
After the evaporation of fuel droplets, further heating of the fuel larks and the rate of chemical reactions are influenced by the temperature, concentration, physical and chemical properties of the reacting substances - fuel and oxygen. When fuel is injected through a nozzle into heated air, the fuel droplets have time to completely evaporate with a large self-ignition delay. Only evaporated fuel burns.
The rate of evaporation of fuel droplets, other things being equal, is directly proportional, and the duration of evaporation is inversely proportional to the pressure of its saturated vapors. Hence, the delay period for self-ignition in the high temperature region will also be inversely proportional to the saturated vapor pressure. Thus, the delay in self-ignition of the fuel seems to completely depend on the physical characteristics. However, there are other views. When burning gas oil and heavy fuel, despite the significant difference in their fractional composition, approximately the same auto-ignition delay periods are obtained. With kerosene, despite the high content of light fractions, there is a significant increase in the delay period of self-ignition, and then pronounced explosive combustion. This allows us to assert that the duration of the ignition delay period at the initial temperatures and pressures observed in diesel engines with self-ignition from compression is determined not only by the physical processes of evaporation and mixture formation, but also by chemical processes reflecting the initial development of the reaction chain. Fuels with a high cetane number have a shorter auto-ignition delay period. This confirms the significant role of the chemical composition of the fuel in organizing the combustion process.
Sokolik believes that during the combustion of fuels there are areas where not only evaporation, but also a chemical reaction is limited, and the temperature coefficient of the chemical reaction can coincide with the temperature coefficient of the evaporation process.
Unlike a spark ignition engine, where a single flame front (focus) is formed, a compression ignition engine produces many flame fronts (foci) from several ignition points in limited volumes.
In the diffusion region, combustion is limited by the period of fuel evaporation. In Fig. Figure 67 shows the duration of complete combustion of kerosene droplets at a combustion rate constant of atomized kerosene in a turbulent flow K = 0.0059 cm2/sec (initial temperature 20 °C, atmospheric pressure). As can be seen from the figure, with sufficiently small droplets, the duration of their combustion is commensurate with the duration of combustion of a homogeneous mixture in a turbulent flame and may even be shorter. In a turbulent flow, the evaporation rate of such droplets can be greater than the vapor combustion rate.
The uniformity of fuel atomization affects not only the ignition delay period, but also the subsequent combustion processes in the engine.
It is believed that effective additives shorten the ignition delay period, i.e., they only affect the initial phase of combustion, which has a preparatory nature. Additives seem to activate the combustion process of fuel.
In the region of low temperatures, the birth of a hot flame is preceded by several preparatory stages, including the formation of a cold flame. At high temperatures, a hot flame arises as a result of the continuous self-acceleration of the reaction through degenerate chain branches that develop into a thermal explosion. For the development of these processes, it is necessary to comply with the appropriate concentration limits of the reacting substances, including atmospheric oxygen.
Many researchers have studied the oxidation processes of fuels in an engine in the period preceding cold-flame combustion and then their self-ignition. It has been established that when fuel oxidizes in an engine with the ignition off, i.e., in the absence of a flame, the entire range of hydrocarbon oxidation products is formed, including a significant amount of peroxides, aldehydes, ketones, and acids. Cetane numbers indirectly characterize the oxidative destruction of hydrocarbons and the rate of their oxidation. In pre-flame reactions, the primary one is the oxidative destruction of hydrocarbons, which occurs under the influence of the heat of the compressed air charge. The temperature in the combustion zone is determined not only by compression, but also by the exothermicity of oxidative reactions.
Combustion is preceded by deep destructive oxidation of hydrocarbons and other organic components of the fuel, mainly in the vapor phase, as a natural preparatory stage for subsequent oxidation, which develops at incomparably high speeds characteristic of combustion.
For the efficient organization of the fuel combustion process, the preparation of the fuel-air mixture plays a very important role. This is especially important for air-breathing engines. This includes proper atomization and dripping of fuel in the primary combustion zone. It is very important to find ways to increase the combustion rate and facilitate ignition of fuel mixtures, especially in . high-altitude systems operating in a low-pressure atmosphere. The use of additives for this purpose is difficult. Their effectiveness depends on the test conditions and on the composition of the working mixture (the presence of a lean or rich mixture). Boron derivatives, in particular (BH4)3Al and B(C2H5)3, turned out to be effective promoters that facilitate the ignition of kerosene at reduced pressure.
To create the best conditions for the combustion process in the engine, careful atomization of the fuel is necessary.
A hydrocarbon droplet with a diameter of up to 45 μm will remain stable in an air flow up to a significant relative speed of ~60 m/sec. Merging of droplets can occur if the distance between them is less than 10 droplet diameters. Viscosity, surface tension, fuel density and air flow have a great influence on the nature of droplet formation in the engine combustion zone.
The evaporation of droplets and the mixing of fuel vapors with air are completed by molecular and turbulent diffusion. Molecular diffusion occurs fairly quickly only over short distances. Turbulent diffusion provides faster formation of a uniform fuel-air flow. In a jet engine, fuel is injected into an air stream, the turbulent nature of which determines good mixing. At the same time, for normal combustion it is very important to maintain the optimal fuel: air ratio. It is difficult to organize combustion if more than double the stoichiometric amount of air moves with the fuel before ignition. The optimal composition of the working mixture is achieved with good mixing of air and fuel.
In jet engines, the duration of fuel evaporation and combustion is less than 0.01 seconds. Evaporation of fuel droplets ends at temperatures up to 370 °C. The flame surrounding a drop of fuel, depending on the oxygen concentration, the chemical composition of the fuel and the size of the drop, can be colorless or luminous. Aromatic hydrocarbons and resins produce a more luminous flame than alkanes.
It is interesting that under comparable conditions, the highest gas temperature at the moment of ignition was characterized by benzene (583 °C); for isoalkanes (2,2- and 2,3-dimethylbutane) this temperature is much lower (443.-446 °C).
The intensity of the flame glow is estimated as the radiating ability of the fuel. The increased radiating ability of the fuel poses a known danger to the normal operation of the engine. In the zone of increased flame radiation, elevated temperatures, local overheating, warping and, finally, possible burnout of the combustion chamber wall occur. The radiating ability of a fuel largely depends on its chemical composition. It has been noticed that at constant engine power, the temperature of the combustion chamber wall decreases in proportion to the increase in hydrogen content in the fuel. This feature is revealed more sharply, the greater the engine power. During combustion, the highest temperature to the walls of a gas turbine engine due to radiation will be imparted by aromatic hydrocarbons that have the lowest relative hydrogen content. The most favorable in this regard will be alkanes characterized by the highest relative hydrogen content. Cyclans will take an intermediate place.
It is believed that in a jet engine the source of the radiating flame, which increases the temperature of the combustion chamber wall, is not the combustion of hydrocarbons directly, but the products of their deep destruction - carbon microparticles. The degree of radiation from a hydrocarbon flame is determined by their chemical structure and the ratio of fuel to air in the working mixture.
The radiating ability of the fuel is assessed either by the height of the non-smoking flame or by the radiation number. In 1960, in the USA, the emission indicator, the luminometric number (ASTM D 1740-60 method), was added to the specifications for jet fuels. This number is determined by the temperature of the flame at a constant intensity of its radiation in the green-yellow band of the visible spectrum (2800-7000 A), recorded by the photocell of the luminometer. The luminometric number, or radiation number (RI), is found from the equation:
where t is the flame temperature of the test fuel, tetralin, and isooctane, respectively, at a constant radiation intensity of the tetralin flame at the smoke point.
For commercial grades of US jet fuels such as kerosene, the radiation number ranges from 45-58, and for fuel of a wide fractional composition JP-4 reaches 70-80.
The luminometric number, which characterizes the radiating ability of the flame during combustion of the fuel mixture, is an improvement on the indicator “height of non-smoking flame”. The device of the luminometer is described in the work. There is a linear relationship between the maximum non-smoking flame height and the radiation number for hydrocarbons. In terms of the height of the non-smoking flame and the number of radiation, normal alkanes are in first place, then alkanes of isomeric structure, mono- and bicyclanes, alkenes and aromatic hydrocarbons.
In the homologous series of hydrocarbons, the radiation number decreases with increasing number of carbon atoms in the molecule. The luminometric number of cyclopentanes is lower than the corresponding cyclohexanes. Thus, the luminometric number of cyclohexane 130 ethylcyclohexane 104, ethylcyclopentane 91, propylcyclopentane 87, isopropylcyclopentane 74, 2-cyclopentylbutane 75, 2-cyclopentylheptane 90.
Below are the luminometric number values for hydrocarbons of various structures:
In Fig. Figure 68 shows the change in the temperature of the combustion chamber wall of gas turbine engines with a power of 160 and 20 hp. With. (efficiency 7.9-9.8 and 17-18.7%, respectively), established after testing 16 fuels of various compositions, which boiled in the range from 26-195 to 137-357 ° C and contained aromatic hydrocarbons 0.4—40.5 volumes. %; alkenes 0–39.6 vol. %, carbon 85.7–87.6 wt. %; hydrogen 12-14.6 wt. %; sulfur 0.002–0.63 wt. %.
In Fig. 69 shows the luminometric characteristics of these fuels depending on their hydrogen content.
The connection between the luminometric number of the fuel, which reflects the radiative activity of the flame, and the chemical structure of the fuel hydrocarbons is quite obvious. Local overheating of the combustion chamber wall, its warping and even burnout are possible not only under the influence of increased flame radiation, but also under the influence of carbon deposits, the thermal conductivity of which is low and approaches the thermal conductivity of the oxides of iron, aluminum and other metals.
In combustion zones where a low concentration of oxygen is established or a mixture over-enriched in fuel accumulates, part of the fuel will undergo thermal decomposition with the formation of incomplete combustion products. These products are highly carbonized substances (95-98% carbon). These include soot and soot deposits. Depending on the speed, pressure and turbulence of the gas flow in the zone of deposit formation, they can have a loose, porous and very compacted structure, be sooty or have a shiny hard surface. The design of the engine fire system significantly affects the amount and nature of deposits, as well as the location of their accumulation.
As mentioned above, deposits deposited on the walls of the combustion chamber, due to their low thermal conductivity, lead to unacceptable local overheating of the surface; deposited on the fuel injector, they change the nature of the supply and distribution of fuel in the combustion zone. Soot particles can rush along with the gas flow from the walls of the flame tubes of a jet engine onto the turbine blades, causing their erosion. All this greatly complicates the operation of the engine.
In table 95 shows data on the relative amount of carbon deposits formed under comparable conditions in an engine with injection and with evaporation. In this case, the amount of carbon deposits obtained during testing of JP-1 fuel (type T-1) is conventionally assumed to be equal to one.
The characteristics of the tested fuels are given in Table. 96. Table data 96 show that the amount of soot increases with increasing aromatic hydrocarbon content in the fuel.
However, we are not talking about all aromatic hydrocarbons, but only about those that boil away at temperatures above 200 °C, which is confirmed by the data given in Table. 97.
However, the content of aromatic hydrocarbons in the fraction boiling above 204°C, apparently, also cannot serve as a final criterion for assessing the carbon-forming ability of the fuel. It should be considered that, in addition to the thermal conditions of the engine, carbon formation is primarily influenced, especially in the initial period of operation, by polycyclic hydrocarbons with the smallest number of side chains, as well as resins, which are the highest molecular weight compounds containing aromatic rings and rings of a heterocyclic structure in the hydrocarbon radical.
Thermal decomposition of fuel proceeds through the stage of formation of pyrolysis products (polycyclic aromatic structures) and their subsequent oxidation. This is confirmed by the presence of a certain amount of oxygen in soot and soot deposits, which is usually more than hydrogen.
Soot and carbon formation apparently occur due to the aggregation of elementary carbon particles formed in the gas flow.
It should be mentioned that some researchers explain the process of direct soot formation in hydrocarbon flames by the droplet theory of combustion. Free hydrocarbon radicals in the process of hydrogenation and condensation first form simple, then more complex high-molecular-weight polycyclic aromatic compounds with low saturated vapor pressure even under flame conditions. Such polycyclic compounds are formed inside a drop of fuel in the form of a core, which, as the shell evaporates, dehydrogenates to form a soot particle.
With an increase in the service life of an aircraft engine, the problem of carbon formation becomes more acute.
The composition of carbon deposits removed from the nozzle and engine swirler after 100 hours of operation on TS-1 fuel, from the nozzle and the walls of the flame tube of the engine combustion chamber after 200 hours of operation on T-2 fuel, and from the flame tube of the engine combustion chamber after 300 hours of operation was studied. on T-1 fuel. All engines operated during the warranty period under operating conditions on airplanes. Fuel injectors and swirlers were heated during operating mode to an average of 250–340 °C, the temperature of the combustion chamber walls in the carbon deposit zone was 250–400 °C, and the gas temperature in front of the turbine for these engines was 500–720 °C. The hardness of the deposits varied. 72-92% of carbon deposits consisted of carbon. Ash elements, mainly iron, silicon and aluminum, turned out to be 0.25-2.8%. The largest amount of them (as well as other metals) was contained in fuels obtained from eastern sulfurous oils. With an increase in sulfur content in fuels, the ash content of soot increased. Oxygen in carbon deposits contained 4.5-22.5%; its content in front of the turbine increased with increasing temperature.
Extraction with chloroform from carbon deposits isolated the ash-free organic part (6-7.5%), containing 80-84% carbon, 9.9-10.0% hydrogen, 2-6% oxygen, 0.6-1.43% sulfur ( with a maximum content of nitrogen in fuels of 0.16%), 0.6-1.44% nitrogen. The oxygen content in soots and the composition of the resins extracted from them give reason to believe that soots are products of pyrolysis and then carbonization of deeply oxidized organic components that make up the fuel. Ash and ash elements contained in carbon deposits are products of high-temperature corrosion of metals and soil dust. All this confirms that the processes of deep oxidative compaction of organic compounds of fuel at 250-400°C are an important source of soot formation. Apparently, by the same mechanism, carbon deposits are formed and smoke occurs in diesel engines.
Using paper chromatography, the presence of more than 90 compounds in the atmosphere contaminated with products of incomplete combustion of diesel fuels was detected, among which were polycyclic aromatic hydrocarbons, phenol and carboxyl derivatives.
The main reason for the drop in power of diesel engines and the appearance of smoke is carbon formation on the injector nozzles. Engine power is restored after cleaning the nozzles. The use of diesel fuels with high thermal stability avoids carbon deposits on injector nozzles. Thus, the thermal stability of diesel fuels during their operation also becomes of great importance, as for jet fuels, although in a slightly different aspect.
The thermal stability of diesel fuels can be assessed by ASTM Method No. 3462 (US Federal Standard), in which the test product is sprayed onto an aluminum plate heated to 260°C. The test lasts three days of 7.5 hours with a daily fuel change. Thermal stability is assessed by the weight gain (in g) of the plates due to the accumulation of deposits on them. Hydrotreated diesel fuel is characterized by higher thermal stability than straight distillation fuels. The service life of injectors when operating on hydrotreated fuel is 2-3 times longer than when operating on straight distilled fuel. In addition, in the first case, smoke is reduced and engine power is increased, and therefore operating costs are reduced.
For gas turbine engines running on distillate diesel fuels, the formation of soot is caused not only by the presence of aromatic hydrocarbons, but also by the increased content of sulfur compounds. Laboratory combustion chamber studies have shown that sulfur content of diesel fuel up to 0.77% has a small effect on the amount of soot formed at the end of combustion, much less than a change in aromatic hydrocarbon content from 6.45 to 23.6%. However, a further increase in the content of sulfur compounds leads to an increase in the density of soot and the sulfur content in it. In general, testing of industrial gas turbines led to the conclusion that changes in sulfur in diesel fuel up to 0.9% and aromatic hydrocarbons up to 25% do not cause a significant increase in carbon deposits on the injectors and in the combustion chamber.
Much attention is paid to finding fuel additives that prevent smoke, carbon and soot formation in the engine combustion zone. Typically, such additives include organometallic compounds containing heavy metals of variable valency (Cu, Fe, Cr, etc.), which increase the rate and depth of oxidation of hydrocarbons during their combustion. Despite ongoing work, such additives have not yet received practical application.
Sequence for determining the specific heat of combustion
The indicator of the specific heat of combustion of kerosene establishes the conditions for its ignition in various devices - from engines to kerosene cutting devices. In the first case, the optimal combination of thermophysical parameters should be determined more carefully. There are usually several schedules for each fuel combination. These graphs can be used to evaluate:
- Optimal ratio of mixture of combustion products.
- Adiabatic flame temperature of combustion reaction.
- Average molecular weight of combustion products.
- Specific heat ratio of combustion products.
This data is necessary to determine the speed of the exhaust gases emitted from the engine, which in turn determines the engine's thrust.
The optimal fuel mixture ratio gives the highest specific impulse of energy and is a function of the pressure at which the engine will operate. An engine with high combustion chamber pressure and low exhaust pressure will have the highest optimum mixture ratio. In turn, the pressure in the combustion chamber and the energy intensity of kerosene fuel depend on the optimal mixture ratio.
In most engine designs using kerosene as fuel, much attention is paid to the conditions of adiabatic compression, when the pressure and volume occupied by the combustible mixture are in constant relationship - this affects the durability of engine elements. In this case, as is known, there is no external heat transfer, which determines the maximum efficiency.
Formulas for calculating volume
The type of formula for calculating the volume of complete combustion products with a theoretically required amount of air depends on the composition of the combustible substance.
Individual chemical compound
In this case, the calculation is carried out based on the combustion reaction equation. The volume of wet combustion products per unit mass (kg) of a combustible substance under normal conditions is calculated using the formula:
Where:
Vp.s. – volume of wet combustion products, m3/kg; – the number of kilomoles of carbon dioxide, water vapor, nitrogen and combustible substances in the combustion reaction equation; M
– mass of flammable substance, numerically equal to molecular weight, kg.
For example, to determine the volume of dry combustion products of 1 kg of acetone under normal conditions, we create an equation for the combustion reaction of acetone in air:
CH3COCH3 + 4O2 + 4 3.76N2 = 3CO2 + 3H2O + 4 3.76N2
Determine the volume of dry combustion products of acetone:
The volume of wet combustion products of 1 m3 of combustible substance (gas) can be calculated using the formula:
Where:
Vp.s. – volume of wet combustion products of 1 m3 of combustible gas, m3/m3; – number of moles of carbon dioxide, water vapor, nitrogen and flammable substance (gas).
Complex mixture of chemical compounds
If the elemental composition of a complex combustible substance is known, then the composition and amount of combustion products of 1 kg of substance can be determined from the equation of the combustion reaction of individual elements. To do this, equations are drawn up for the combustion reaction of carbon, hydrogen, sulfur and the volume of combustion products per 1 kg of combustible substance is determined. The combustion reaction equation has the form:
C + O2+ 3.76N2 = CO2 + 3.76N2
When 1 kg of carbon is burned, 22.4 / 12 = 1.86 m3 CO2 and 22.4 × 3.76/12 = 7.0 m3 N2 are obtained.
The volume (in m3) of combustion products of 1 kg of sulfur and hydrogen is determined in the same way. The data obtained is shown below:
| CO2 | N2 | H2O | SO2 | |
| Carbon | 1,86 | 7,00 | – | – |
| Hydrogen | – | 21,00 | 11,2 | – |
| Sulfur | – | 2,63 | – | 0,7 |
When carbon, hydrogen and sulfur burn, oxygen comes from the air. However, the combustible substance may contain oxygen, which also takes part in combustion. In this case, correspondingly less air is consumed for combustion of the substance.
The combustible substance may contain nitrogen and moisture, which during the combustion process become combustion products. To account for them, it is necessary to know the volume of 1 kg of nitrogen and water vapor under normal conditions.
The volume of 1 kg of nitrogen is 0.8 m3, and the volume of water vapor is 1.24 m3. In air at 0 °C and a pressure of 101325 Pa, per 1 kg of oxygen there is 3.76 × 22.4 / 32 = 2.63 m3 of nitrogen.
Based on the given data, the composition and volume of combustion products of 1 kg of combustible substance are determined.
For example, to determine the volume and composition of wet combustion products of 1 kg of coal, consisting of 75.8% C, 3.8% H, 2.8% O, 1.1% N, 2.5% S, W = 3 .8%, A = 11.0%.
The volume of combustion products will be as follows, m3:
| Composition of combustion products | CO2 | H2O | N2 | SO2 |
| Carbon | 1,86 × 0,758 = 1,4 | – | 7 × 0,758 = 5,306 | – |
| Hydrogen | – | 11,2 × 0,038 = 0,425 | 21 × 0,038 = 0,798 | – |
| Sulfur | – | – | 2,63 × 0,025 = 0,658 | 0,7 × 0,025 = 0,017 |
| Nitrogen in combustible substance | – | – | 0,8 × 0,011 = 0,0088 | – |
| Moisture in a flammable substance | – | 1,24 × 0,03 = 0,037 | – | – |
| Sum | 1,4 | 0,462 | 6,7708 – 0,0736 = 6,6972 | 0,017 |
From the total volume of nitrogen, the volume of nitrogen attributable to oxygen in the composition of coal is subtracted 0.028 × 2.63 = 0.0736 m3. The result indicates the composition of the combustion products of coal: the volume of wet combustion products of 1 kg of coal is equal to:
Vp.s. = 1.4 + 0.462 + 6.6972 + 0.017 = 8.576 m3/kg.
Mixture of gases
The amount and composition of combustion products for a mixture of gases is determined by the equation of the combustion reaction of the components that make up the mixture. For example, methane combustion proceeds according to the following equation:
CH4 + 2O2 + 2 × 3.76N2 = CO2 + 2H2O + 7.52N2
According to this equation, the combustion of 1 m3 of methane produces 1 m3 of carbon dioxide, 2 m3 of water vapor and 7.52 m3 of nitrogen. The volume (in m3) of combustion products of 1 m3 of various gases is determined similarly:
| CO2 | H2O | N2 | SO2 | |
| Hydrogen | – | 1,0 | 1,88 | – |
| Carbon monoxide | 1,0 | – | 1,88 | – |
| Hydrogen sulfide | – | 1,0 | 5,64 | 1,0 |
| Methane | 1,0 | 2,0 | 7,52 | – |
| Acetylene | 2,0 | 1,0 | 9,54 | – |
| Ethylene | 2,0 | 2,0 | 11,28 | – |
Based on the given figures, the composition and quantity of combustion products of the gas mixture are determined.
Analysis of combustion products taken from fires in various rooms shows that they always contain a significant amount of oxygen. If a fire occurs in a room with closed window and door openings, then the fire in the presence of fuel can continue until the oxygen content in the mixture of air with combustion products in the room decreases to 14-16% (vol.). Consequently, during fires in enclosed spaces, the oxygen content in combustion products can range from 21 to 14% (vol.). The composition of combustion products during fires in rooms with open openings (basement, attic) shows that the oxygen content in them can be below 14% (vol.):
| CO | CO2 | O2 | |
| In the basements | 0,15-0,5 | 0,8-8,5 | 10,6-19 |
| In the attics | 0,1-0,6 | 0,3-4,0 | 16,0-20,2 |
Based on the oxygen content in combustion products during fires, one can judge the coefficient of excess air at which combustion occurred.
Application of the substance
The fuel we know best is kerosene. The petroleum product is used as jet fuel in rockets and aircraft. This is also a well-known fuel used in firing porcelain and glass products. Kerosene is also produced for household lighting and heating devices. Used for metal cutting machines. It is also a solvent (for example, for applying pesticides), a raw material in oil refining.
Kerosene can actually be used as a substitute for Arctic and winter fuel. But in this case, it is not an equivalent alternative - it is necessary to add cetane-increasing and anti-wear additives. For multi-fuel engines (based on a diesel engine), it is possible to use pure kerosene, but only for a short time.
In winter, it will be acceptable to add 20% kerosene to summer diesel fuel in order to reduce the pour point of the latter. In this case, performance characteristics will not suffer.
As for the entertainment sector, here it is kerosene that acts as the main fuel when holding various fire shows (performances with the “participation” of fire). This is facilitated by its excellent absorbency and relatively low combustion temperature. In everyday life, kerosene is known to be used as a means to remove rust and wash various mechanisms.
What do planes fuel?
There are two types of aircraft fuel. Piston engines that power small airplanes and helicopters run on gasoline, just like car engines. True, the composition of such fuel is somewhat different from automobile fuel. The gas turbine engines (turbojet and turboprop) that power virtually all commercial aircraft today consume jet fuel, also called jet fuel.
The main brand of aviation kerosene, which in Russia fuels almost all passenger, transport and military subsonic aircraft and most helicopters, is TS-1 - sulfur fuel. It is produced from oil with a high sulfur content.
In Europe, the basis of the aviation fuel supply system is Jet A-1 kerosene. It is considered more environmentally friendly precisely due to its lower sulfur content - during its production, the straight-run kerosene-legroin fraction undergoes a complete hydrotreating procedure. Russian jet fuel is a mixture of hydrotreated and unrefined straight-run distillates. In general, these are analogues - moreover, the domestic product can be used at much lower temperatures than Jet. Today, the TS-1, along with the Jet A-1, is included in international documents and operating manuals not only for Russian-made aircraft, but also for aircraft of the Airbus and Boeing families (though only those operating flights within Russia). But this is jet fuel for civil aviation, not intended for supersonic aircraft.
Gazprom Neft has launched R&D to create unleaded aviation gasoline. Together with scientists from the All-Russian Research Institute of the Petroleum Industry, the company’s specialists began developing a formulation of unleaded fuel with an octane rating of 91 in 2014, and this work has now been completed.
The main aviation fuel for supersonic aviation is RT. During its production, aggressive and unstable compounds containing sulfur, nitrogen and oxygen are removed from petroleum distillate using hydrotreating
At the same time, the thermal stability of the fuel increases, which is extremely important when flying at supersonic speeds, when friction with the air heats up the entire aircraft body, and with it the fuel in the tanks
Of course, RT, which has such characteristics, can be used in conventional aircraft instead of TS-1. For the fastest aircraft, T-6 jet fuel is used, which has even greater thermal stability and increased density.
As for aviation gasoline, it is essentially automobile motor fuel, but with improved properties that affect engine reliability. It is the need to increase detonation resistance, octane number, grade, providing a reserve of dynamic characteristics and reliability, that forces aviation gasoline manufacturers to add tetraethyl lead to it (lead). Due to its toxicity, this additive has long been banned in the production of motor gasoline, but an airplane engine operates under much more intense conditions, and no one has yet succeeded in creating unleaded aviation gasoline that is not inferior in performance to leaded gasoline, the octane number of which exceeds 92–95.
At the same time, the most modern and advanced airplanes and helicopters with piston engines require aviation gasoline with a high octane number - no less than 100. Therefore, leading manufacturers and research centers around the world are now developing environmentally friendly analogues of leaded aviation gasoline 100LL (one of the most popular brands in the world). Gazprom Neft also has a similar program.
100 thousand flights operate around the world every day
Main directions in the use of kerosene
Kerosene is used in various fields. There are several main groups for its use:
Aviation fuel
Kerosene became widespread at a time when active development of turboprop and jet technology began throughout the world. The substance turned out to be the most suitable fuel. The reason is quite simple. The octane number of kerosene does not reach 50. This property made it ideal for refueling aircraft.
Jet fuel is one of the most common types of petroleum products.
Solvent and cleaner in production
There is another fairly popular type of this fuel - dearomatized or deep hydrogenation. The composition and characteristics of the raw materials are suitable for the production of polyvinyl chloride. Kerosene is used as a solvent. To use it for flushing, special additives are added to the substance. They prevent the accumulation of electrical charges.
Fuel and lubricant for household use
As in ancient times, lighting fixtures are fueled with kerosene. It is also suitable for incandescent lamps. Also among the areas of application of the substance:
- Metal cutting.
- Impregnation of leather.
- Dissolving varnish coatings, etc.
When choosing kerosene for any application, it is important that the mass of sulfur in the composition is minimal. This makes the raw materials safe for humans and the environment
To effectively use raw materials as fuel, it is important to take into account such indicators as:
- Turbidity level.
- Mass of kerosene.
- Height of non-smoking flame.
- Flash point.
They are considered especially carefully in aviation. This is due to the fact that flights take place at high altitudes. Since the atmosphere is at a very low temperature, it is important that the fuel does not transform into crystalline form.
If you want to learn more about the composition and characteristics of kerosene, or choose a specific type for any application, please contact our managers. Call, fuel specialists will help you understand all the nuances!
Composition and characteristics of kerosene, main properties of different types
07.02.2018
The properties of kerosene have made it in demand in various fields. A transparent, oily liquid suitable for use as fuel, fuels and lubricants and all kinds of additives. Kerosene is resistant to low temperatures and has high combustion and evaporation rates. It is also compatible with raw materials having a different composition.
Kerosene, a petroleum product obtained by rectification and recycling of raw materials. In some cases, it is additionally hydrotreated
Composition and properties of kerosene
Kerosene, the composition and properties of which are suitable for creating jet fuel, refueling various devices and washing mechanisms, is characterized by a high degree of pumpability. It is also in demand due to the absence of neoplasms and deposits.
Kerosene as a fuel has a wide range of uses, from rockets to firing chambers and lighting devices
The method of processing raw materials affects the content of various impurities. It may contain oxygen, sulfur and nitrogen compounds. The number of hydrocarbons is indicated as a percentage:
- Unlimited – up to 2.
- Aromatic – from 5 to 25.
- Naphthenic – from 20 to 50.
- Aliphatic – from 20 to 60.
At different t, the fractional composition of kerosene changes its volume. For 20°C and 25°C – 200%, for 80°C – 270%. Proper splitting of a complex component mixture into separate parts is carried out based on the properties of the oil products.
Extract of kerosene indicators in accordance with GOST 4753-68
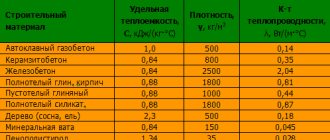
Basic indicators of the physical properties of kerosene
The physical properties of kerosene have many sub-items. The basic ones include those that affect the quality and scope of the substance.
Kerosene density
The degree of density is a widely used characteristic of petroleum products. To determine it, a relative value is used. So at 20°C, it will reach from 780 to 850 kg/m3. When calculating, the temperature of the substance, the actual density of the product and distilled water are important.
The color of kerosene varies from yellowish to light brown, it can also be colorless
Kinematic viscosity of kerosene
The composition of kerosene determines its viscosity. Moreover, the higher the temperature of the substance, the lower this indicator. The characteristic under consideration is reflected in:
- Properties of fuel systems operation.
- The quality of the mixture formed.
- Combustion processes in an engine.
At 20°C the viscosity level will be 1.2 - 4.5 mm2/s.
In order for kerosene to serve as an arctic fuel, additives must be added to it to increase the cetane number and reduce engine wear
Flash point of kerosene
The chemical composition of kerosene is reflected in its flash point. The value of the indicator from 28°C to 60°C determines the fire safety level of the substance. All standards are regulated by current GOSTs.
Heat from burning kerosene
The characteristic under consideration demonstrates the amount of heat released during absolute combustion of a mass unit of raw material. For kerosene the figure ranges from 42.9 to 43.1 MJ/kg.
At what temperature kerosene becomes cloudy can be determined optically. To do this, changes in the ability of a substance to transmit light rays are recorded.
Chemical properties of kerosene
Kerosene - the chemical properties of the fuel, such as volatility and flammability, depend on the composition of the raw material and the type of its processing. The concentration of aromatic hydrocarbons is different, which led to the following kerosene groups:
- Aviation . In turn, it is divided into jet (RT) and aircraft (TS-1) fuel. Used to lubricate fuel systems in engines of various aircraft. The refrigerant also plays a role. Has increased thermal oxidation and combustion mark. Characterized by stability and resistance to low temperatures.
- Technical . All tolerances are regulated by GOST “Kerosene for technical purposes” 18499-73. Grades KT-1 and KT-2 replace solvents or cleaners for washing components and spare parts of vehicles, equipment and mechanisms.
- Lighting . Types KO-25, 25 or 30 are used to refill kerosene lamps. Some types of fuel are used to impregnate tanned leather. Among the advantages is the absence of soot and soot during combustion.
Important technical characteristics of kerosene include increased volatility. vapors in the air up to 300 mg/m3 are not dangerous to humans. When working with fuel, it is also necessary to take into account its high level of flammability - combustion at t° 57°C, self-ignition at t° 216°C.
Kerosene is often used to wash mechanisms and remove rust.
If you need kerosene, you can find out the characteristics of various types from the specialists of TC AMOX. The optimal option will be selected based on the purpose of application. Pay attention to the fuel catalog, which presents common types of kerosene, diesel fuel, gasoline and fuels and lubricants. Call us, we will answer all your questions!
Fill out the feedback form, our managers will contact you!
Main areas of use
In conclusion, we present the most common areas of use of the substance:
- Aviation kerosene. This is the name of motor fuel for gas turbine engines, which are equipped with various aircraft. These are kerosene fractions of direct distillation of oil. They are often hydrotreated and additives are added to improve performance properties. In Russia, five varieties of such fuel are produced for subsonic aviation - TS-1, T-1, T-1S, T-2 and RT, and for supersonic aviation - two (T-6 and T-8V).
- Rocket kerosene. Here this petroleum product acts as a hydrocarbon, environmentally friendly fuel and the working fluid of hydraulic machines. Its use in rocket engines was proposed back in 1914 by Tsiolkovsky. Paired with liquid oxygen, it is used in the lower stages of many launch vehicles.
- Technical kerosene. This is a raw material for the production of aromatic hydrocarbons, ethylene, propylene. In addition, it is the main fuel for firing porcelain and glass, and a solvent for washing parts and mechanisms.
- Lighting kerosene (KO-25, KO-30, KO-20, KO-22). It is used in lighting fixtures and is used as fuel for some kitchen stoves (kerosene stoves, kerosene stoves, kerosene gas). Another use is in heating. This is a solvent, a cleaning agent (widely used to remove residues of thermal pastes, various paints and varnishes), and a degreaser.
- Automotive kerosene. This application was characteristic of the dawn of the development of internal combustion engines. The petroleum product was widely used as fuel for carburetor and diesel internal combustion engines.
Among the non-trivial uses, the following can be distinguished: a folk remedy for getting rid of lice, treating head lice and diphtheria. In addition, kerosene helped get rid of bedbugs when wiping furniture with it.
As you have seen, kerosene immediately determines a complex of characteristics. And this seems natural given its multiple uses.
Jet fuels
Main article: Jet fuel
Kerosene is a fraction of oil that boils away mainly in the temperature range of 200-300 ° C. Jet fuel, fuel for aviation jet engines, are usually kerosene fractions obtained by direct distillation from low-sulfur (for example, T-1) and sulfur (TS-1 ) oils. Currently, straight-run aviation fuel is scarce; hydrotreating and additives are widely used.
Kerosene is used for domestic purposes as heating and motor fuel, a solvent for varnishes and paints. Jet fuel is used as fuel for gas turbine engines of airplanes and helicopters of civil and military aviation, and in addition, fuel on board an aircraft can also be used as a coolant or coolant (fuel-air and fuel-oil radiators), and as a working fluid hydraulic systems (for example, controlling the cross-section of an engine jet nozzle). Jet fuels are also widely used as a solvent during aircraft maintenance and when cleaning contaminants manually or mechanically (for example, in an ultrasonic installation for cleaning filters, jet fuel is used as a working fluid). Aviation jet fuels undergo a total of up to 8 levels of quality control, and in the Russian Federation, in addition, acceptance by a military representative.
For jet fuels, the main quality indicators are:
- mass and volumetric heat of combustion
- fuel thermal stability
- saturated steam pressure
- kinematic viscosity
- compatibility with structural and sealing materials
- carbon deposit and anti-wear properties
- electrical conductivity
- sulfur
- acidity
Jet fuels are produced mainly from middle distillate fractions of oil, boiling at a temperature of 140-280 C° (naphtha-kerosene). Wide-fraction grades of jet fuels are produced using gasoline fractions of oil in the processing. To obtain some types of jet fuels (T-8B, T-6), vacuum gas oil and secondary oil refining products are used as raw materials.
Jet fuels consist of 96-99% hydrocarbons, which are divided into three main groups:
- paraffin
- naphthenic
- aromatic.
In addition to hydrocarbons, jet fuels contain small amounts of sulfur, oxygen, nitrogen, organometallic compounds and resinous substances. Their content in jet fuels is regulated by standards.
In Russia and the CIS countries operating Soviet aircraft, the following types of aviation fuel are used:
— TS-1
in the Russian Federation it is produced according to GOST 10227-86 as amended. 1-6. — straight-run fraction 150-250 C°, or a mixture of straight-run and hydrotreated fractions (the main limitation is the content of total sulfur and mercaptan no more than 0.2% and 0.003%). The most widespread type of aviation fuel in the Russian Federation and the post-Soviet space, intended for all old types of turboprop and subsonic turbojet engines, it is also used by aircraft of foreign manufacturers. In terms of its characteristics and scope of application, it approximately corresponds to foreign Jet-A kerosene. It is a backup in relation to RT fuel.
— RT
— high-quality fuel, oil fraction 135-280 C° with complete hydrotreating. Sulfur content: total - 0.1%, mercaptan - 0.001%. Due to hydrocracking, the fuel is “dry”, that is, it has low lubricating properties. During the production process, antioxidant and anti-wear additives are introduced into it. Intended for turbojet subsonic and some supersonic aircraft (Su-27, Tu-22M3, etc.), as well as as a fuel reserve for TS-1. There are no foreign analogues for this fuel.
—T-6
and
T-8B
- heat-resistant jet fuel for the engines of some supersonic aircraft (for example,). They are produced using a very complex technology with hydrotreating and the introduction of additives. These fuels are produced only for the needs of the Russian Ministry of Defense.
Technical kerosene
Technical kerosene is used as a raw material for the pyrolytic production of ethylene, propylene and aromatic hydrocarbons, as a fuel mainly for firing glass and porcelain products, and as a solvent for washing mechanisms and parts. Kerosene, dearomatized by deep hydrogenation (contains no more than 7% aromatic hydrocarbons), is a solvent in the production of PVC by polymerization in solution. Additives containing magnesium and chromium salts are added to kerosene used in washing machines to prevent the accumulation of static electricity charges. In Russia, standards for technical kerosene are set by GOST 18499-73 “Kerosene for technical purposes”
Quality of kerosene in Russia and abroad
Aviation kerosene must satisfy a number of requirements to ensure reliable engine operation and operational requirements. Aviation kerosene must have good volatility, allowing to obtain a homogeneous air-fuel mixture of optimal composition; have a group hydrocarbon composition that ensures a stable, detonation-free combustion process; do not change its composition during storage and do not have a harmful effect on the parts.
Russian and foreign requirements for kerosene differ. Domestic aviation kerosene has a higher crystallization temperature. Standards also differ in the content of hydrocarbons and sulfur. Foreign jet fuel has a higher flash point and is less fire hazardous, but starting engines using this fuel is much more difficult. The crystallization temperature of Russian kerosene does not exceed minus 55-60°C, while for foreign kerosene producers this parameter is up to 50°C.
There are minor complaints from foreign fuel producers and consumers against Russian producers. Firstly, this is low thermal stability, and secondly, an insufficient level of anti-wear qualities. But in general, the quality of Russian kerosene meets international standards. Dozens of samples of aviation kerosene produced by Russian factories were tested. This examination showed the compliance of Russian kerosene, in particular thermal stability, with foreign standards for such fuel grades as TS-1, RT and JetA-1. This means that Russian kerosene has standardized quality.