Basic properties of metals
TO
category:
Metals
Basic properties of metals
Next: Tensile Testing
The properties of metals are divided into physical, chemical, mechanical and technological.
Physical properties include: color, specific gravity, fusibility, electrical conductivity, magnetic properties, thermal conductivity, heat capacity, expansion when heated.
Chemical properties include oxidation, solubility and corrosion resistance.
Mechanical - strength, hardness, elasticity, viscosity, plasticity.
Technological ones include hardenability, fluidity, malleability, weldability, and machinability.
Let us give brief definitions of mechanical properties.
The strength of a metal is its ability to resist external forces without breaking.
Hardness is the ability of a body to resist the penetration of another, harder body.
Elasticity is the property of a metal to restore its shape after the cessation of the action of external forces that caused a change in shape (deformation).
Toughness is the ability of a metal to resist rapidly increasing (impact) external forces. Viscosity is the opposite property of brittleness.
Plasticity is the property of a metal to deform without destruction under the influence of external forces and retain a new shape after the force ceases. Plasticity is the inverse property of elasticity.
Modern methods of testing metals are mechanical tests, chemical analysis, spectral analysis, metallographic and radiographic analyses, technological tests, flaw detection. These tests make it possible to gain an understanding of the nature of metals, their structure, composition and properties, as well as to determine the good quality of finished products.
Mechanical testing is of critical importance in industry.
Parts of machines, mechanisms and structures operate under loads. Loads on parts come in different types: some parts are loaded with a force constantly acting in one direction, others are subject to impacts, and in others the forces change more or less often in their magnitude and direction. Some machine parts are subject to stress at elevated temperatures, corrosion, etc.; Such parts work in difficult conditions.
In accordance with this, various methods of testing metals have been developed, with the help of which mechanical properties are determined.
The most common tests are static tensile testing, dynamic testing and hardness testing.
Static tests are those in which the metal being tested is subjected to a constant force or a force that increases very slowly.
Dynamic tests are those in which the metal being tested is subjected to an impact or force that increases very quickly,
In addition, in some cases, fatigue, creep and wear tests are performed, which provide a more complete picture of the properties of metals.
Mechanical properties. The first requirement for any product is sufficient strength.
Metals have higher strength compared to other materials, so loaded parts of machines, mechanisms and structures are usually made of metals.
Many products, in addition to general strength, must also have special properties characteristic of the operation of this product. For example, cutting tools must have high hardness. Tool steels and alloys are used for the manufacture of cutting and other tools.
For the manufacture of springs and springs, special steels and alloys with high elasticity are used.
Viscous metals are used in cases where parts are subject to shock loads during operation.
The plasticity of metals makes it possible to process them by pressure (forging, rolling).
Physical properties. In aircraft, automobile, and railroad car manufacturing, the weight of parts is often the most important characteristic, so aluminum and magnesium alloys are especially useful here. Specific strength (the ratio of tensile strength to specific gravity) for some, such as aluminum alloys, is higher than for mild steel.
Fusibility is used to produce castings by pouring molten metal into molds. Low-melting metals (for example, lead) are used as quenching media for steel. Some complex alloys have such a low melting point that they melt in hot water. Such alloys are used for casting printing matrices, in devices used to protect against fires, etc.
Metals with high electrical conductivity are used in electrical engineering, for the construction of power lines, and alloys with high electrical resistance are used for incandescent lamps and electric heating devices.
The magnetic properties of metals play a primary role in electrical engineering (dynamos, electric motors, transformers), in electrical instrument making (telephone and telegraph devices), etc.
The thermal conductivity of metals makes it possible to heat them uniformly for pressure treatment and heat treatment; it also provides the possibility of soldering metals, welding them, etc.
Some metal alloys have a linear expansion coefficient close to zero; such alloys are used for the manufacture of precision instruments, radio tubes, etc. The expansion of metals must be taken into account when constructing long structures, such as bridges. It should also be taken into account that two parts made of metals with different expansion coefficients and fastened together can bend and even break when heated.
Chemical properties. Corrosion resistance is especially important for products operating in highly oxidized environments (grids, machine parts in the chemical industry). To achieve high corrosion resistance, special stainless, acid-resistant and heat-resistant steels are produced, and protective coatings are also used for products.
Technological properties. Technological properties are very important in the production of certain technological operations.
—
All materials have a number of properties that differ in physical, mechanical, chemical and technological.
The physical properties of metals include specific gravity, melting point, color, electrical conductivity, thermal conductivity, heat capacity, expansion when heated, magnetic properties and some others. Depending on the operating conditions or operation of the parts, some of these properties become critical and serve as the basis for the choice of material in the manufacture and use of the part. For example, specific gravity and strength are important qualities for a material in aircraft construction, where lightweight and durable parts are needed. The melting point is of great importance for parts operating at high temperatures, for example, filaments in electric lamps, lining of melting furnaces, etc. Therefore, aircraft parts are made from aluminum and magnesium alloys, and tungsten, etc. is used to make filaments.
Of the chemical properties of metals, the most important are corrosion resistance, as well as oxidation and solubility.
A very important role in determining the suitability of a metal as a material for machine parts and mechanisms is played by its mechanical properties.
Mechanical properties: strength, hardness, elasticity, ductility, toughness and brittleness.
Strength is the ability of a material to resist forces without collapsing or changing its acceptable shape.
An example of a durable material is steel. Steel products are difficult to break and change shape. Unlike steel, mercury does not have strength. At ordinary temperatures it is in a liquid state and does not retain its shape.
Hardness is the ability of a material to resist the penetration of another, harder body into it. The hardest substance known to us is diamond. Various types of steel and so-called hard alloys have high hardness. Hardness is the most important property of the materials from which cutting tools are made.
Elasticity is the ability of a body to restore its original shape after the cessation of the forces that caused this change. An example of an elastic body is a steel spring, which, after the cessation of the impact forces, restores its previous shape.
Plasticity is the ability of a material to change its shape under the influence of forces without collapsing and not to restore its previous shape after the cessation of the forces. An example of a ductile metal is lead. This quality is essentially opposite to elasticity.
Viscosity is the ability of a material to withstand mechanical stress (impact) without breaking. Very tough, for example, low-carbon steel, used for non-critical parts.
Fragility is a quality opposite to viscosity, the ability of a body to easily collapse under mechanical influences (impacts). An example of a brittle metal is cast iron.
The technological properties of metals and alloys are a combination of various mechanical and physical properties that manifest themselves in the manufacturing processes of machine parts.
The technological properties of the metal include the ability to be processed by cutting, casting, rolling, forging, drawing, the ability to be welded and subject to heat treatment.
To determine the properties of metals and alloys, they use: a) mechanical tests, which establish their strength, hardness, elasticity, ductility, toughness and fragility; b) physical measurements of specific gravity, melting point, heat and electrical conductivity; c) chemical analysis, which determines the qualitative and quantitative composition of the alloy; d) metallographic analysis, which allows one to obtain data on the structure and properties of the metal using a microscope and an X-ray machine; e) technological tests, which make it possible to determine the suitability of the metal for a given type of processing.
Physical, chemical, mechanical and technological properties of metals
To choose the right material for a specific purpose, you need to know the properties of metals. For example, the manufacture of cutting tools requires strong, hard and wear-resistant metal materials.
The physical properties of metals and alloys are determined by color, specific gravity, density, melting point, thermal expansion, thermal and electrical conductivity, and magnetic properties.
The physical properties of metals are characterized by certain numerical values, which are given in Table 1.
Table 1
Physical properties of some metals
| Metal | Symbol | Color | Density, kg/m3 | Melting point, °C | Udel. electrical resistance at 20 °C, 10-6 Ohm∙m |
| Aluminum | Al | Silver white | 2700 | 658,7 | 0,029 |
| Tungsten | W | Brilliant white | 19300 | 3380 | 0,053 |
| Iron | Fe | Silver white | 7800 | 1539 | 0,100 |
| Cobalt | Co | Silver white | 8900 | 1490 | 0,062 |
| Magnesium | Mg | Shiny silver white | 1700 | 650 | 0,047 |
| Copper | Cu | Red | 8900 | 1083 | 0,017 |
| Nickel | Ni | Silver-white with a grayish tint | 8900 | 1452 | 0,070 |
| Tin | Sn | Silver white | 7300 | 231,9 | 0,124 |
| Lead | Pb | Slate gray | 11400 | 327,4 | 0,220 |
| Titanium | Ti | Silver white | 4500 | 1668 | 0,470 |
| Chromium | Cr | Brilliant grayish white | 7100 | 1550 | 0,150 |
| Zinc | Zn | Slate gray | 7100 | 419,5 | 0,060 |
The ratio of a body's mass to its volume is a constant value for a given substance and is called density.
Density and specific gravity are of great importance when choosing metal materials for the manufacture of various products. Thus, parts and structures in instrument making, aircraft and carriage manufacturing, along with high strength, must have low density. Of the metals most widely used in technology, magnesium and aluminum have the lowest density.
All metals, as bodies of a crystalline structure, transform at a certain temperature from solid to liquid and vice versa. The temperature at which a metal changes from solid to liquid is called the melting point.
Melting point is an important physical property of metals. Knowledge of the melting point of metals and alloys is necessary in metallurgy, foundry, hot metal forming, welding, soldering and other processes involving heating of metal materials.
The ability of metals to transfer heat from more heated parts of the body to less heated ones is called thermal conductivity.
Among metallic materials, silver, copper, and aluminum have the best thermal conductivity. These same metals are also the best conductors of electric current.
The thermal conductivity of metals is of great practical importance. Metals and alloys with high thermal conductivity are used to make machine parts that absorb or release heat during operation.
Metals and alloys with low thermal conductivity require slow and prolonged heating to fully warm up. Rapid heating and rapid cooling of such metal materials can cause cracks to form. This must be taken into account when heat treating, hot working, metal casting, etc.
Various substances, including metals, expand when heated and contract when cooled. The difference in the magnitude of the thermal linear expansion of materials is characterized by the linear expansion coefficient α, which shows by what fraction of the original length l0 at 0 °C the body elongated due to its heating by 1 °C. The unit of measurement for α is °C-1.
The thermal expansion of metals must be taken into account in the manufacture and operation of precision measuring instruments and tools, the manufacture of foundry molds, hot metal forming and in other cases associated with heating and cooling.
Parts of precision instruments and measuring instruments are made of materials with a low coefficient of linear expansion; parts of automatically operating mechanisms, which, when extended, must close an electrical circuit, are made of materials with a high coefficient of linear expansion.
Electrical conductivity is the ability of metals to conduct electric current.
High electrical conductivity is possessed by those metals that conduct electric current well, that is, without heat loss.
Magnetic properties . Some metals become magnetized when exposed to a magnetic field. After removing the magnetic field, they have residual magnetism. This phenomenon was first discovered on iron and was called ferromagnetism. Iron, nickel, cobalt and their alloys have strong magnetic properties. The metal materials listed above are called ferromagnetic . Other metals and alloys have extremely weak magnetic properties, so in practice they are considered non-magnetic.
Magnetic transformations are not associated with changes in the crystal lattice or microstructure; they are caused by changes in the nature of the electronic interaction.
Magnetic permeability is the ability of metals to become magnetized under the influence of a magnetic field.
When heated, the ferromagnetic properties of the metal decrease gradually: at first weakly, then sharply, and at a certain temperature (Curie point) they disappear (the Curie point for iron is 768 ° C, for nickel - 360 ° C, for cobalt - 1130 ° C). Above this temperature, metals become paramagnetic (weakly magnetic materials).
The chemical properties of metals include their ability to resist chemical or electrochemical effects of various environments (corrosion) at normal and high temperatures.
The physical properties of metals discussed above are revealed in phenomena that are not accompanied by a change in the substance. For example, heating metals or passing electric current through metals is not accompanied by chemical changes. During chemical phenomena, metals are transformed into other substances with different properties.
Many metals undergo chemical changes under the influence of the external environment, that is, they are destroyed by corrosion. A measure of corrosion resistance is the rate of propagation of metal corrosion in a given environment and under given conditions: the lower this speed, the more corrosion-resistant the metal.
Nickel, titanium and their alloys have high corrosion resistance in the atmosphere and in aggressive environments. Titanium and its alloys are close to noble metals in corrosion resistance.
Strength is the ability of a material to resist external forces without destruction.
Elasticity is the ability of a material to restore its original shape and size after the cessation of the external forces that caused the deformation.
Plasticity is the ability of a material to change its shape and size under the influence of external forces without collapsing, and to retain the resulting deformations after the cessation of external forces.
The mechanical properties of metals are a set of properties that characterize the ability of metallic materials to resist external forces (loads).
The mechanical properties of metallic materials include: strength, hardness, ductility, elasticity, toughness, brittleness, fatigue, creep and wear resistance.
Hardness is the ability of a metal to resist the penetration of another, harder body into it.
Strength is the ability of a metal to resist destruction under the influence of external forces.
To determine the strength, a metal sample of a specified shape and size is tested at the highest tensile stress, which is called ultimate strength (temporary resistance).
Plasticity is the ability of a metal, without breaking, to change shape under load and retain it after the load is removed.
Toughness is the ability of a metal to resist rapidly increasing (shock) loads.
The technological properties of metals and alloys characterize their ability to be amenable to various methods of hot and cold working. The technological properties of metals and alloys include casting properties, malleability, weldability, machinability with cutting tools, and hardenability.
The machinability of metals is characterized by their mechanical properties: hardness, strength, ductility.
Performance properties characterize the ability of a material to work under specific conditions.
Wear resistance is the ability of a material to resist surface destruction under the influence of external friction.
Corrosion resistance is the ability of a material to resist the action of aggressive acidic and alkaline environments.
Heat resistance is the ability of a material to resist oxidation in a gas environment at high temperatures.
Heat resistance is the ability of a material to retain its properties at high temperatures.
Cold resistance is the ability of a material to retain plastic properties at subzero temperatures. Cold brittleness is the tendency of a metal to transform into a brittle state as the temperature decreases. Cold brittle are iron, tungsten, zinc and other metals that have a body-centered cubic and hexagonal close-packed crystal lattice.
Red brittleness is the tendency of a metal to transform into a brittle state with increasing temperature.
When choosing a material to create a structure, it is necessary to fully take into account the mechanical, technological and operational properties.
Crystalline and amorphous substances
Gkl or gvl, which is better: differences, what is the difference, what to choose?
When describing the physical properties of solids, it is customary to describe the structure of the substance. If you examine a sample of table salt under a magnifying glass, you will notice that the salt consists of many tiny crystals. In salt deposits you can also find very large crystals. Crystals are solid bodies shaped like regular polyhedra. Crystals can have different shapes and sizes. The crystals of some substances, such as table salt, are fragile and easy to destroy. There are crystals that are quite hard. For example, diamond is considered one of the hardest minerals. If you examine table salt crystals under a microscope, you will notice that they all have a similar structure. If we consider, for example, glass particles, they will all have a different structure - such substances are called amorphous. Amorphous substances include glass, starch, amber, and beeswax. Amorphous substances are substances that do not have a crystalline structure
Other areas of application
Do you know that copper is very often used as a catalyst for the polymerization of acetylene? Due to this property, copper pipelines used to transport acetylene are allowed to be used only when the copper content in them does not exceed 64%.
People have learned to use the malleability of copper in architecture. Facades and roofs made from the finest copper sheets serve trouble-free for 150 years. This phenomenon is explained simply: in copper sheets, the corrosion process auto-extinguishes. In Russia, copper sheets are used for facades and roofs in accordance with the norms of the Federal Code of Rules SP 31-116-2006.
In the not-too-distant future, people are planning to use copper as germicidal surfaces in clinics to prevent bacteria from moving indoors. All surfaces that are touched by the human hand - doors, handles, railings, water stop fittings, countertops, beds - specialists will make only from this amazing metal.
Effect of temperature on properties
The effect of temperature is ambiguous. Low-carbon and medium-carbon steels become more ductile with increasing temperature (1). High-alloy steels have greater cold ductility (2). For ball bearing steels, ductility is practically independent of temperature (3). Individual alloys may have a range of increased ductility (4). Industrial iron in the range of 800...1000C is characterized by a decrease in plastic properties (5). At temperatures close to the melting point, ductility decreases sharply due to possible overheating and burnout.
Story
One of the first metals that people began to actively use in their households was copper. Indeed, it is too accessible to obtain from ore and has a low melting point. For a long time, the human race has known the seven metals, which also includes copper. In nature, this element is found much more often than silver, gold or iron. Ancient objects made of copper, slag, are evidence of its smelting from ores. They were discovered during excavations in the village of Çatalhöyük. It is known that in the Copper Age, copper things became widespread. In world history it follows the stone one.
S. A. Semenov and his colleagues conducted experimental studies in which they found that copper tools are superior to stone ones in many respects. They have a higher speed of planing, drilling, cutting and sawing wood. And processing bone with a copper knife takes the same amount of time as with a stone one. But copper is considered a soft metal.
Very often in ancient times, instead of copper, its alloy with tin was used - bronze. It was necessary for the manufacture of weapons and other things. So, the Bronze Age replaced the Copper Age. Bronze was first obtained in the Middle East in 3000 BC. BC: people liked the strength and excellent malleability of copper. The resulting bronze was used to make magnificent tools for labor and hunting, dishes, and jewelry. All these items are found in archaeological excavations. Then the Bronze Age gave way to the Iron Age.
How could copper be obtained in ancient times? Initially, it was mined not from sulfide, but from malachite ore. Indeed, in this case there was no need to engage in pre-firing. To do this, a mixture of coal and ore was placed in a clay vessel. The vessel was placed in a shallow pit and the mixture was set on fire. Then carbon monoxide began to be released, which contributed to the reduction of malachite to free copper.
It is known that copper mines were built in Cyprus already in the third millennium BC, where copper smelting was carried out.
On the lands of Russia and neighboring states, copper mines arose two millennia BC. e. Their ruins are found in the Urals, and in Ukraine, and in Transcaucasia, and in Altai, and in distant Siberia.
Industrial copper smelting was developed in the thirteenth century. And in the fifteenth, the Cannon Yard was created in Moscow. It was there that guns of various calibers were cast from bronze. An incredible amount of copper went into making bells. In 1586 the Tsar Cannon was cast from bronze, in 1735 the Tsar Bell was cast, and in 1782 the Bronze Horseman was created. In 752, craftsmen made a magnificent statue of the Big Buddha at Todai-ji Temple. In general, the list of works of foundry art can be continued endlessly.
In the eighteenth century, man discovered electricity. It was then that huge volumes of copper began to be used to make wires and similar products. In the twentieth century, they learned to make wires from aluminum, but copper was still of great importance in electrical engineering.
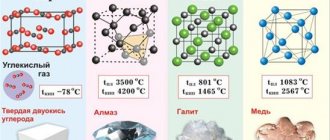
Where is copper found in nature?
The Earth's crust contains (4.7-5.5) x 10-3% copper (by mass). In river and sea water it is much less: 10-7% and 3 x 10-7% (by mass), respectively.
Copper compounds are very often found in nature. In industry, chalcopyrite CuFeS2, called copper pyrite, bornite Cu5FeS4, and chalcocite Cu2S are used. At the same time, people also find other copper minerals: cuprite Cu2O, azurite Cu3(CO3)2(OH)2, malachite Cu2CO3(OH)2 and covellite CuS. Very often the mass of individual copper accumulations reaches 400 tons. Copper sulfides are formed mainly in hydrothermal medium-temperature veins. Copper deposits can often be found in sedimentary rocks - shales and cuprous sandstones. The most famous deposits are in the Transbaikal region Udokan, Zhezkazgan in Kazakhstan, Mansfeld in Germany and the honey belt of Central Africa. Other richest copper deposits are located in Chile (Colhausi and Escondida) and the USA (Morenci).
Most copper ore is mined by open pit mining. It contains from 0.3 to 1.0% copper.
Copper marking
What grades of copper does a person use to produce the products he needs? There are many of them: M00, M0, M1, M2, M3. In general, grades of copper are identified by the purity of its content.
For example, copper grades M1p, M2p and M3p contain 0.04% phosphorus and 0.01% oxygen, and grades M1, M2 and M3 - 0.05-0.08% oxygen. There is no oxygen in the M0b brand, and in MO its percentage is 0.02%.
So, let's take a closer look at copper. The table below will provide more accurate information:
| Copper grade | M00 | M0 | M0b | M1 | M1r | M2 | M2r | M3 | M3r | M4 |
| Percentage copper | 99,99 | 99,95 | 99,97 | 99,90 | 99,70 | 99,70 | 99,50 | 99,50 | 99,50 | 99,00 |