Metals and alloys are literally the basis of human civilization. Pure metals are not very often used in the national economy, but alloys are used everywhere. This is not surprising, since the alloy combines the properties of several substances in the best proportion. This article talks about the production and processing of molten brass, preparation of the material, composition, properties and application of the material.
Classification of brasses
Since other alloying elements are added to the alloy of copper and zinc, they are distinguished:
- two-component alloys
- and multicomponent brass alloys.
Alloying elements of complex alloys: Mg, Sn, Ni, Pb, Si, Fe, Al and others. All of them affect the properties of products in a certain way. Mg in combination with Fe and Al affects the strength characteristics and corrosion resistance. Ni – has a positive effect on resistance to oxidative processes. Pb increases the ductility and malleability of brass. Such alloys are often used by artisans; such alloys are also called automatic alloys, because they lend themselves well to machine processing. Si is controversial, but it affects the strength characteristics of the alloy, and in combination with Pb it can compete for primacy with tin bronze in terms of antifriction qualities.
No less important is the classification of Cu and Zn alloys according to the method of their processing. There are:
- casting alloys,
- pressure-processed alloys,
- You can also include special brasses in this group.
Brass with a high zinc content is better suited to hot pressure treatment at temperatures from 300 to 700°C, however, as the Zn concentration increases above 30%, the ductility and strength of the alloys decreases, so in practice alloys with a Zn content higher than 39% are not used for these purposes. . When cold, any brass alloys can be processed well.
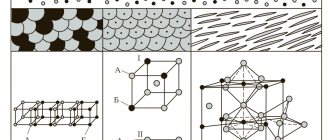
It has already been said about the difference in the phase states of brass, but to complete the picture it should be defined again:
- a-phase brass
- and b-phase brass.
The first - with a Zn content of up to 39%, the second (two-phase) - higher. Brass in the a-phase has higher ductility and strength than in the b phase, since two-phase alloys tend to exfoliate due to the fact that copper and zinc will not form a strong bond.
Since brasses vary in zinc content, it is also customary to distinguish:
- red
- and yellow brass
The zinc content in red brass (tombaka) ranges from 5 to 20%, and in yellow brass it is more than 20%. The higher the Zn content in the composition, the lower the cost of the alloy.
Physical and chemical properties
Brass has no formula as it is an alloy. It has a high melting point (about 900°C), but increasing the amount of zinc can reduce it.
The density is 8.6 g/cm3, which is slightly lower than the specific gravity of copper, the main component of the brass alloy. Has the highest thermal conductivity.
The specific heat capacity is lower than that of copper (0.377 J/(kg*K)), so the ability to crack is reduced.
It is characterized by the ability to polish, pressure treatment, welding, resistance to aggressive atmospheric environments, and resistance to very low temperatures.
Compared to bronze, copper-zinc metal has worse anti-corrosion and strength, but relative to copper they are better.
There are two phases: α (with a cubic cell in the crystal structure) and β.
Properties of alloys
To understand how different alloying compositions and proportions affect the qualities of brass, below we have provided several tables and diagrams. But first, let's look at the principles of marking brass. In Russia, two-component alloys are marked with the letter L and numbers indicating the percentage of copper by chemical composition. (L80 contains 79-81% Cu, up to 0.3% impurities and Zn in the remainder). Multicomponent alloys are also marked with the letter L, after which the letters of the alloying elements are indicated, followed by numerical designations indicating the percentage of copper and alloying compositions, in the order indicated by the letters (LA77-2 - 77% Cu, 2% Al).
Advantages of joining brass parts using argon-arc welding
It is not for nothing that experts consider argon arc welding the best method for joining brass parts. It has many advantages, among which the most significant are versatility (you can connect both small and large elements) and process safety. There are others:
- no flux or special expensive electrodes are needed;
- environmental friendliness;
- high speed of work;
- the seam is not only durable, but also aesthetic;
- absence of slag on the weld surface;
- oxide and nitride crusts do not appear during the welding process;
- inert gas supplied to the junction of parts displaces dangerous compounds arising from the oxidation of zinc.
Inert gas allows you to protect the cleaned edge of a metal part from the influence of external air. The argon flow prevents the formation of slag, crusts of oxides and nitrides, and harmful chemical compounds.
Application
The range of brasses produced in Russia is very large. There are about 37 main grades of two and multi-component alloys, not counting special and secondary brasses. So the range of their applications is extremely wide and will be discussed in more detail in articles devoted to specific alloys. However, examples of the use of brasses within the above classification can be given.
Two-component wrought brass alloys L96-L80 are used mainly for the production of parts in the chemical industry, radiator and capillary tubes, thermal equipment, and in mechanical engineering. L68-L60 - used in the manufacture of stamped products, fittings and fasteners, parts in the automotive industry, condenser pipes, branch pipes.
The scope of application of multicomponent deformable alloys is much wider and fits into the production framework of such industries as: shipbuilding, chemical industry, mechanical engineering, production of thermal equipment, precision instruments, aviation industry and others. It is noteworthy that multicomponent wrought alloys are mainly used for the production of small parts with good antifriction properties.
Casting brass alloys are used for the manufacture of critical parts and structural elements. They have great strength. They are used to cast fittings, make nuts, worm screws, as well as bearings, bushings and corrosion-resistant parts.
Composition and structure
There are two types of brass alloys:
- Multicomponent.
- Two-component.
Multi-component brasses, in addition to zinc and copper, may include other non-metals and metals. They have a significant impact on the final properties of the alloy. Thus, adding tin to the composition increases the resistance of the material to sea water. And the addition of, for example, nickel, increases the strength of the brass product.
Two-component alloys typically include zinc and copper in varying proportions.
Another classification method relates to processing methods:
- Deformable brass can be bent, rolled, and given different shapes even at home. These alloys are produced in the form of wire, rods and sheets, which are subsequently used, for example, in the production of pipes.
- Casting alloys can only deform when exposed to high temperatures and significant pressure. This technology is used to make automobile parts, bearings, etc.
Story
Brass production was practiced in ancient Rome, and later in Egypt, Greece and China. According to information presented in SBIE, the ancient Romans made brass by fusing copper and the mineral gallium in the form of carbon ZnCO3. The purity of such an alloy was not great, so real high-quality brass appeared much later, when in 1746 Andreas Sigismund Maggraf found a way to extract pure zinc by calcining zinc oxide in a retort made of refractory clay without access to air, and condensing the zinc in the gas phase in a refrigerator.
The word zinc goes back to the German zinke (tooth), probably this name is associated with the shape of sphalerite crystals (zinc blende) from which zinc was subsequently mined on an industrial scale. Sphalerite contains zinc sulfide ZnS. Concentrates are obtained from it using a pyrometallurgical scheme. First, the mineral is crushed and then placed in a selective flotation apparatus, where other concentrates are recovered along with the zinc concentrate. Next, zinc concentrates are enriched and reduced by roasting in a fluidized bed, and then by sintering. The distillation method is not currently used to obtain pure zinc. The most widely used method in our time is the hydrometallurgical method of producing Zn by electrolysis.
Equipment for casting brass - Metalworker's Handbook
Copper is one of the first metals mastered by mankind.
Copper
Thanks to its low melting point and high plasticity, it has not lost its popularity for the fifth millennium. Red metal is widely used both in industry and at home to make jewelry, crafts and parts by casting copper.
In industrial settings, technologies such as
Copper casting
- Casting copper into molds
- Powder metallurgy
- Electroplating
- Hot and cold rolled
- Sheet stamping
- Wire drawing
- Mechanical restoration
They require complex and expensive professional equipment, highly qualified personnel and are accompanied by high energy costs.
Wire drawing of copper
At home, in a small workshop, simple technologies are used, largely repeating the work techniques of the Copper Age masters. This includes copper casting and wire drawing, as well as forging and embossing. Despite the simplicity and antiquity of technological techniques, home craftsmen achieve high quality products. Sufficient casting accuracy is ensured by careful production of the mold.
Characteristics of copper
Copper is a metal with a relatively low melting point (1083C), a density of 8 g/cm3 and high ductility. It occurs in nature in the form of nuggets.
Thanks to these qualities, it became the first metal mastered by mankind. Archaeologists have found tools and weapons in burials dating back to the 3rd millennium BC.
Most likely, humanity mastered copper casting even earlier, at the end of the Stone Age.
Basic properties of metals of the copper subgroup
The Latin name of the metal, Cuprum, is associated with the name of the island of Cyprus, a famous ancient center for the production of bronze products. Copper-based alloys - bronze and brass - have high strength and are less susceptible to oxidation. Bronze was widely used as the main metal of mankind until the development of mass steel production technologies.
Copper has excellent electrical and thermal conductivity. This leads to its widespread use in electrical and thermal engineering.
In addition, copper has pronounced bactericidal properties.
Copper smelting and casting equipment
Casting copper at home does not require particularly complex or expensive equipment. Buying it or making it yourself is quite within the capabilities of a home craftsman.
Required
- Crucibles are cylindrical open vessels.
Examples of graphite crucibles
- Steel tongs for removing and placing the crucible in the furnace.
- Muffle furnace or gas burner.
- Steel hook for removing oxide crust from the surface of the melt.
- Casting mold.
First of all, you need to melt the copper. The better the feedstock is crushed, the faster the melting will occur. Melting will occur in a crucible made of ceramic or refractory clay.
The muffle furnace must be equipped with a thermometer and a glass window for visual inspection.
Electronic temperature control and maintenance system will make copper casting easier and provide better casting quality.
Molds for copper casting are made based on the model. Depending on the chosen technology, the molds can be disposable (from a mixture specially molded in the formwork) or reusable - steel molds. Recently, molds made from high-temperature silicone have become widespread.
At home, disposable forms are more often used. The model is made from wax or special types of plasticine. The model completely repeats the spatial configuration of the future product. When a hot melt is poured into a mold, the wax melts and is replaced by metal, which takes its place and repeats all the details of the mold's relief. This form is called lost wax.
Burnout mold for copper casting
There are also burnt forms. They use a model made of flammable material, such as papier-mâché. In this case, the model burns when a high-temperature melt is poured, the combustion products in the form of gases exit through the filling hole.
Application of copper casting
Copper casting is used to manufacture a wide range of products. In jewelry, the legendary metal is often used as part of alloys. It is added in small quantities to gold items to increase their strength and abrasion resistance. Bronze, which is an alloy of copper and tin, is used to create designer pendants, chains, rings and earrings.
Copper jewelry
Copper casting is also used to make fishing lures of unique shapes. Another area of application is the creation of original scale models of equipment - ships, cars, tanks, airplanes, etc. Here, in addition to bronze, brass is used - an alloy with zinc.
Brass and bronze are also used for casting decorative elements of rooms, linings and designer door handles. Here, in addition to the structural advantages - strength, durability and appearance, the bactericidal properties of copper and its alloys are also used.
The process of melting copper at home
The process of casting copper at home is not difficult, but requires careful preparation, planning and strict adherence to time and temperature parameters.
It begins with grinding wire or scrap and placing it in a crucible. At the same time, turn on the muffle furnace to warm up. The better the metal is crushed, the faster and more efficiently both the melt and the casting will proceed.
It is important to monitor the melt temperature. When the temperature is exceeded, the melt begins to actively absorb oxygen from the air and oxidize, which leads to a decrease in the quality of the castings.
To reduce the influence of atmospheric oxygen, the surface of the melt is sprinkled with crushed activated carbon.
If a muffle furnace is not available, the crucible can be placed on a welded tripod and heated with a gas burner with the nozzle turned upward.
Important! The burner must be securely fastened
You can also make a stove out of fireclay bricks and a steel grate on which coal will be scattered. Such a stove must be blown with a powerful fan or vacuum cleaner.
After the metal has melted, you need to securely grab the crucible with tongs and remove it from the furnace, placing it on a refractory base.
Using a steel hook, you need to move the film of oxides formed on the surface of the melt towards the wall, and, without allowing it to cool, pour it in a thin stream into the hole of the mold. If a lost wax mold is used, ensure that the stream of metal being poured allows the model material to escape.
Be sure to allow the casting to cool completely before disassembling the mold, cleaning and finishing the product.
Important! The use of safety glasses and gloves with gaiter is mandatory. Do not forget to check the availability and functionality of a fire extinguisher suitable for extinguishing live electrical installations.
Let your casting be successful, and the copper casting made at home will delight you and your customers!
, please select a piece of text and press Ctrl+Enter.
Bronze casting: artistic bronze casting technology
Bronze casting allows us to produce products that have exceptional decorative appeal. The technology of casting from this alloy, which is based on copper, has been known for many centuries, but even in our time it continues to be improved.
The appearance of bronze products speaks of the painstaking work of the master, turning faceless metal into a work of art
Brass production
Brass production is a complex technological process that involves the copper and zinc industries, as well as recycling methods. As raw materials for producing alloys, we use blanks of copper, zinc and other metals for multicomponent alloys manufactured in accordance with GOST, as well as our own production waste and secondary raw materials.
Brass is obtained by melting these raw materials in electric arc furnaces or solid fuel furnaces in crucibles, or even without crucibles in reverberatory furnaces. The raw materials are pre-prepared and the ovens are also cleaned. Copper is heated to red heat and placed in the furnace first, after which zinc lumps are added. To obtain complex alloys, copper is also added first, after which the remaining elements are added.
Methods of obtaining
The brass production process requires the availability of special raw materials - copper, zinc, as well as other metal blanks (lead, aluminum, silicon, nickel), which serve as alloying additives.
The components are placed in a melting furnace with a hood. Copper must first be red-hot, pieces of zinc are added to it, then additives.
Liquid metal is poured into molds to produce casting alloys. If they are subjected to deformation, the output will be deformable brass.
Rolled metal and castings
After obtaining a homogeneous mass, the alloy is poured into molds, if it is cast brass, and from it the following is obtained:
- flat ingots
- and round ingots.
Deformable alloys, after casting into molds, undergo a deformation procedure. From them they produce: brass strip, brass plates, brass wire, brass pipes, brass circles, brass sheets, brass rods.
The resulting products may vary in the degree of additional processing (hardening, aging), as well as in the state of the material (soft, semi-hard, hard and extra-hard). Additional thermal preparation of workpieces can significantly increase their corrosion resistance and strength.
Advantages and disadvantages
Every metal has special properties that can be attributed to both advantages and disadvantages. It all depends on the situation. Brass is used extremely rarely in construction, which is more evidence of the relevance of other materials, and not at all of the disadvantages of the alloy.
The main advantage of brass is its low weight, which makes the material popular in rocket and aircraft construction. In domestic conditions, this is only necessary in situations where, for example, a light water supply system is needed.
The alloy also has excellent decorative properties. It has a very varied and attractive color palette. Accessories and fittings, household and decorative items made with brass will always be beautiful, emphasizing the luxury and elegance of the interior. At the same time, the color of brass remains for a very long time.
The material also has low thermal conductivity , which is often used for the manufacture of systems and objects for which heat retention is very important. We are talking about the production of bathtubs or furniture.
Brass is a diamagnetic metal, meaning it will be pushed out of any magnetic field. This alloy has been used for the production of compass frames for a long time. Now this quality is actively used in instrument making.
The corrosion resistance of the material is even greater than that of ordinary copper, but it decreases greatly as temperatures increase. Therefore, it is very beneficial to use brass pipes for water supply. For the heating system, it is still better to take a copper pipeline.