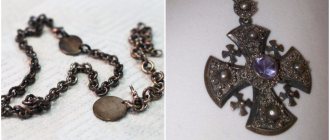How to coat metal so it doesn't rust?
There are several ways to coat metal surfaces to prevent them from rusting. ... The most popular converters today are the following:
- "Zinkor". The composition forms a thin layer on metal objects. ...
- "VSN-1". Serves as a corrosion neutralizer in small areas. ...
- "B-52". ...
- Berner. ...
- "SF-1".
19 Dec.
2022 Interesting materials:
How to measure a blood pressure cuff? How to measure one meter? How to measure a wig? How to measure the height of iPhone 12 PRO? How to measure the width of your step? How to measure your height accurately? How to measure temperature with a regular thermometer? How to measure thickness? How to measure the thickness of metal with a caliper? How was the drum invented?
Loaded loads. I don't know how to paint it. Help!
#1 Message dadon » 11-05-2006 07:41 –> Added: 11-05-2006 07:41 Message title: Loaded cargo. I don't know how to paint it. Help!
Is there a technology for covering lead at home? I want black weights.
#2 Message by Plyushkin » 11-05-2006 07:53 –> Added: 11-05-2006 07:53 Message title:
#3 Message dadon » 11-05-2006 08:26 –> Added: 11-05-2006 08:26 Message title:
2Plyushkin: dear, if you have nothing to write, don’t mess with the topic. write in PM.
A specific question was asked - I want to paint it, I don’t know how.
#4 Message Volan » 11-05-2006 08:31 –> Added: 11-05-2006 08:31 Message title:
#5 Message Sergeich » 11-05-2006 08:39 –> Added: 11-05-2006 08:39 Message title:
#6 Message dadon » 11-05-2006 08:51 –> Added: 11-05-2006 08:51 Message title:
#7 Message by alexfast » 11-05-2006 08:58 –> Added: 11-05-2006 08:58 Message title:
#8 Message by slavik » 11-05-2006 09:12 –> Added: 11-05-2006 09:12 Message title:
#9 Message wetfoxes » 11-05-2006 09:41 –> Added: 11-05-2006 09:41 Message title:
#10 Message Vigorous Lin » 11-05-2006 10:26 –> Added: 11-05-2006 10:26 Message title:
#11 Message dadon » 11-05-2006 10:44 –> Added: 11-05-2006 10:44 Message title:
#12 Message Vurdalak » 11-05-2006 11:05 –> Added: 11-05-2006 11:05 Message title:
Or maybe not paint them? Lead darkens over time - it oxidizes. The color, of course, will not be black, but gray for sure!
And the question is: I’ve heard so many times about heat-shrink tubing, but where can I get it? If sold in stores, which ones?
#13 Message Vigorous Lin » 05/11/2006 11:15 –> Added: 05/11/2006 11:15 Post title:
dadon Well, you need to ask the Taimenevites, in the community of the apox website.. I suspect that they were simply painted with something like enamel.
Vurdalak You don’t have to paint it. And take it and cover it with an oxide film of a pleasant dark gray color with a bluish tint. To do this, you can use my “know-how” (at least, I thought of this on my own) - rub the weights with a slurry of water and Kommet powder and leave for a few minutes. The coating is dense and does not change in sea water (tested). And updating and refreshing it, if necessary, is a matter of literally a few minutes, you don’t even need to remove weights from your belt!
#14 Message Vurdalak » 11-05-2006 11:34 –> Added: 11-05-2006 11:34 Message title:
#15 Message Stas » 11-05-2006 12:01 –> Added: 11-05-2006 12:01 Message title:
#16 Message dadon » 11-05-2006 12:41 –> Added: 11-05-2006 12:41 Message title:
#17 Message PioNeeR » 11-05-2006 17:29 –> Added: 11-05-2006 17:29 Message title:
#18 Message Stas » 11-05-2006 18:17 –> Added: 11-05-2006 18:17 Message title:
1 held, (dipped and dried in two thin layers), now I don’t have them, let them use them - they drowned them. Now I use shot (buckshot) in nylon bags. 2 no photos.
the price of doubt is 100 rubles, buy a sealant and try it.
#19 Message by Plyushkin » 05/22/2006 15:11 –> Added: 05/22/2006 15:11 Message title:
Sorry if I was rude.
Let me explain my idea. (although everything has already been said above, I just have to repeat myself) potentially the weight belt is a consumable. and you need to be mentally prepared to leave him. (it doesn’t matter that I personally know of one case when a hunter was on the verge of dropping loads) However, this piece of equipment should be as cheap as possible. All the coatings on loads that I have seen peel off/break off over time. Once I saw rubberized weights. the color was light green. price - well, I don’t need cargo for that kind of money. Lead weights also darken over time and accumulate a huge number of scratches and dents, becoming completely unsightly. but nevertheless they continue to perform their functions. during night swims, a completely black weight belt is a possible extra headache (and where is it? I put it here) also the color of the weights does not affect how conveniently the weight is placed on the swimmer’s body. I don’t see the point in inventing something with loads. They just have to be. in my case, 6 weights of 2 kg each on a rubber weight belt are enough. and I don’t care what they are, and I think the fish I hunt care even more than me.
#20 Message Wad Moscow » 05/22/2006 19:26 –> Added: 05/22/2006 19:26 Message title:
Let's get started
As we have already said, for antique metal processing, it is better to choose a fine sunny day and perform all operations in the open air. But if this is not possible, then make sure that the room where you work is well ventilated or at least ventilated. The process of artificial “rusting” of iron consists of several stages:
- Thoroughly clean the iron object from dust, oils and other contaminants. If its surface is painted, the paint coating should be removed mechanically or chemically.
- Sand the metal surface with sandpaper until it is slightly rough. This will make the metal oxidation reaction more active.
- Place a plastic container on a flat base and place the prepared part in it.
- Protect your eyes with goggles and your hands with rubber gloves. By neglecting protective measures, you expose your body to serious danger.
- Apply vinegar to the item placed in the container using a spray bottle.
- Allow the acid to react with the metal. The appearance of rust will become noticeable within a few minutes.
- Mix two cups of hydrogen peroxide, four tablespoons of vinegar and one and a half teaspoons of table salt in a plastic bottle. Attach the sprayer to the container and begin applying the resulting mixture to the metal. If the proportions are maintained correctly, then the reaction will be instantaneous - bubbles will appear on the surface of the metal, and the rust that has already begun to appear after the initial treatment will become even more active.
- Leave the product to dry with the composition applied to it for 5-10 minutes. If a larger area is being processed, it may take a little longer.
- In order to get a light touch of antiquity, a single treatment is enough. But if you need to get a very rusty thing, the process will have to be repeated 2-4 times.
- After achieving the expected result, remove the item from the plastic container, and dilute the remaining solution in it with water and pour it down the drain.
- To preserve the resulting rust effect, apply a clear acrylic sealant to the “aged” surface. The protective layer will not only prevent rust from staining objects in contact with the part, but also stop the corrosion process, which can continue without your participation and, in the end, completely destroy the item.
Metal aging technology
- Before starting work, the metal must be cleaned of any contaminants. The success of the work depends on the preliminary preparation, so it is necessary to clean the surface diligently. Sometimes it is even recommended to anneal the metal to remove residual resins or other substances.
- If the parts are made of copper or brass, then it must first be bleached with a weak solution of sulfuric acid. And iron is treated with a more concentrated solution of sulfuric acid. Aluminum products are treated with bicarbonate of soda. Regardless of the processing method, after completion of work, the parts must be thoroughly rinsed under running water and cleaned with a stiff brush.
- Nitric acid is used to work with brass and copper. Since the fumes of this acid are harmful to humans, special care should be taken, and in general it is not recommended to work with this substance indoors. It is better to go outside to work with metal.
- The acid is applied to the surface of the metal product with a cotton swab wound on a wooden stick. The reaction to the substance will be noticeable immediately - the surface of the metal will change color from rich green to black. After the reaction has occurred, the metal must be heated until the composition completely evaporates. Then the product is washed under running hot running water.
- The color of the finished product can range from olive green to brown and from light gray to black. This depends on the concentration of the acid, the duration of contact with it, and the degree of heating. The resulting effect is quite durable, so you can polish or grind the part.
- If iron parts are aged, they are thoroughly cleaned, coated with drying oil, and then heated to 300-400 degrees Celsius. For uniform surface treatment, it is better to heat the part in an oven. If a light tone of the part is required, then nitric acid is used.
- To process aluminum products I use kerosene or soot. These substances emphasize the structure of this metal and give it a beautiful decorative appearance.
Lead corrosion in water
This is one of the materials that does not begin to deteriorate when in contact with water. This is true for both fresh and sea water. But it is also worth remembering that lead should not be allowed to come into contact with drinking liquid - it can be deposited in the human body and stimulate the destruction of bone tissue.
The risk of rust becomes much higher with strong aeration of water. In this case, the corrosion intensity per day is at least 8-9 grams per square meter.
How to “age” metal
At all times, rust has been one of the most serious enemies of iron products.
It is very difficult for things damaged by corrosion to regain their attractiveness, and in particularly advanced cases, an item corroded by rust has to be thrown away. Meanwhile, as strange as it may sound, a red or brown coating on metal can be a welcome phenomenon. Designers, restorers and other specialists who, by the nature of their activities, come across antiques and their imitation, know this better than anyone else. The need to “age” objects and their individual parts made of iron and its alloys does not arise very rarely. For example, you have become the owner of a wonderful vintage chest, which is worthy of becoming a real decoration for your living room or office, but has a serious drawback - the absence of one of the lid hinges. Today, finding or ordering an identical element from a locksmith is not at all difficult, but even a skillfully made replica will not look next to the original, which has a noble antique appearance with a slight touch of corrosion. Is it possible to process metal so that it looks tens, or even hundreds of years older? Of course you can and we will tell you how to do it without much effort and expense.
Method for rapid aging of metal products
Often, for various creative ideas, antique buttons, needles, pins, nails, horseshoes, etc. are needed. You can quickly age such metal products using vinegar (1/4 cup) and salt (2 tablespoons).
- Mix both substances and immerse the metal part in the resulting solution.
- The composition is mixed, covered with a lid with holes and left for 12 hours.
- Then the parts are taken out and dried on newspaper.
- If excess rust has formed, you can sand it off with sandpaper.
Metal aging technology
- Before starting work, the metal must be cleaned of any contaminants. The success of the work depends on the preliminary preparation, so it is necessary to clean the surface diligently. Sometimes it is even recommended to anneal the metal to remove residual resins or other substances.
- If the parts are made of copper or brass, then it must first be bleached with a weak solution of sulfuric acid. And iron is treated with a more concentrated solution of sulfuric acid. Aluminum products are treated with bicarbonate of soda. Regardless of the processing method, after completion of work, the parts must be thoroughly rinsed under running water and cleaned with a stiff brush.
Materials and tools
To “aging” metal objects, you do not need either expensive equipment or complex, multi-component chemical compositions. Almost everything you need for work can be found in a toolbox, garage or, as a last resort, in the nearest hardware store:
It is better to do the work outdoors on a sunny day, and this is due not only to the use of reagents that give a violent reaction with the release of gases, but also to the fact that under the influence of direct sunlight the work will progress much faster.