Alloy steels
Elements whose content exceeds the normal limit specified in the standards are called alloying additives. Changing the chemical composition of a metal by introducing alloying additives is called steel alloying. Main purposes of alloying:
- increased hardenability;
- obtaining specific strength properties;
- inducing desired structural changes;
- obtaining special chemical or physical properties;
- improvement and simplification of heat treatment technology;
- increasing corrosion resistance and resistance to various temperatures.
Based on the above, it follows that steel alloying is a metallurgical smelting process during which various additives are introduced into it. Alloying elements are added in two ways:
- Volumetric - components penetrate into the deep structure of the material by adding them to the charge or melt.
- Surface - introduction of alloying components only to the top layer of steel, to a depth of 1-2 mm. This method gives the material certain properties, for example, anti-friction.
What elements can be included in steels?
The elements that make up steels can be divided into three groups:
- The first includes the basic elements that are necessarily present in all steels: iron and carbon.
- The second one contains impurities. They, in turn, can be divided into three more groups:
- Phosphorus, sulfur, silicon and manganese have different effects on the properties of steels, but they are always present in small quantities, so they are classified as constant.
- Oxygen, hydrogen and nitrogen are also present in all steels, but they are all undesirable and negatively affect the properties. They are classified as hidden.
- Arsenic, copper, zinc, lead, tin and a number of other elements are not found in every grade of steel. Their presence is a feature of the deposits where ore is mined. Such impurities are considered random.
- The third group includes alloying elements: chromium, vanadium, molybdenum, tungsten and others. They are purposefully added to steel to obtain the desired properties.
Alloying elements
- Chrome – increases strength and hardness, increases toughness. It is added to tool steels to increase hardenability. In the case of stainless steels, it determines corrosion resistance.
- Nickel – increases strength and hardness while maintaining high toughness. Reduces the threshold temperature of brittleness. This affects the good hardenability of steels, especially with the participation of chromium and molybdenum.
- Manganese - increases hardness and strength due to plastic properties. Manganese steel is characterized by an increased elastic limit and higher abrasion resistance.
- Silicon plays the role of a deoxidizing agent in the metallurgical process. Its addition increases the strength and hardness of steel.
- Molybdenum increases the hardenability of steels more than chromium and tungsten. Reduces the fragility of metal after high tempering.
- Aluminum – strongly deoxidizes, prevents the growth of austenite grains.
- Titanium – reduces grain size, which leads to greater resistance to splits and cracks. Improves susceptibility to metalworking.
There can be several alloying additives, and to obtain certain characteristics, their introduction can be carried out at different stages of smelting.
In addition to the fact that various additives are introduced into the steel composition, the material itself also contains impurities that cannot be completely removed from the composition:
- Carbon – contributes to increased hardness, strength and impact resistance. However, its excess in the metal composition reduces ductility and all of the above characteristics.
- Manganese is a deoxidizing agent that protects against oxygen and sulfur.
- Sulfur – its content is considered high if it is above 0.6%, which has a bad effect on ductility, strength, weldability and corrosion resistance.
- Phosphorus - leads to increased fluidity and fragility, reduces viscosity and ductility.
- Oxygen, nitrogen, hydrogen - make the alloy more brittle and reduce its endurance.
The influence of chromium on the properties of steels
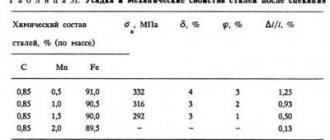
The tendency of chromium to form carbides is average among other carbide-forming alloying elements. At a low Cr/C ratio of chromium content relative to iron, only cementite of the type (Fe,Cr)3C is formed. With an increase in the ratio of chromium to carbon content in Cr/C steel, chromium carbides of the form (Cr,Fe)7C3 or (Cr,Fe)23C6 or both appear. Chromium increases the ability of steels to be thermally hardened, their resistance to corrosion and oxidation, provides increased strength at elevated temperatures, and also increases the abrasive wear resistance of high-carbon steels.
Chromium carbides are also wear-resistant. They are the ones who provide durability to steel blades - it’s not for nothing that knife blades are made from chrome steels. Complex chromium-iron carbides enter the solid solution of austenite very slowly - therefore, when heating such steels for hardening, a longer exposure at the heating temperature is required. Chromium is rightfully considered the most important alloying element in steels. The addition of chromium to steels causes impurities such as phosphorus, tin, antimony and arsenic to segregate to the grain boundaries, which can cause temper brittleness in steels.
Application
Due to such characteristics as strength, load resistance, hardness, reduced magnetization and the desired level of toughness, alloy steel is used in a wide variety of areas of human activity. From it they produce:
- medical instruments, including cutting ones;
- parts with high supporting and radial loads;
- elements of metalworking machines;
- stainless steel utensils;
- car parts;
- aerospace parts;
- molds and other elements for hot stamping that retain their properties at temperatures up to + 600 degrees;
- measuring instruments and so on.
Welding alloys
We noted that after adding components, metalworking, including using a welding machine, becomes more difficult. Let's see what the features are.
Low alloy
- The seam must not be allowed to cool quickly - then microcracks may appear.
- The device must be with reverse polarity and constant voltage.
- Calcium fluoride coated electrodes must be used.
- The process is without interruption, smoothly at an average speed of 20 m/h.
- Voltage – 40 V and current – 80 A.
Medium alloyed
- The electrodes should contain less alloying substances than the alloy.
- If the sheet is wider than 5 mm, use argon welding.
- For a gas appliance, use a mixture of acetylene and oxygen.
High alloy
- Thermal capture of the material is minimal.
- Electrodes with calcium fluoride coating.
- Do not use gas welding.
In the article we told you everything about alloy steel: what it means, the features of its production, properties and composition. We hope that the information was informative for you.
After you read the article, you can read about our products. for 15 years on the Russian market. During this time, we covered almost all cities of the country.
Source
Classification of alloy steels
Taking the principle of separation according to the structure formed under conditions of slow cooling of the steel in the temperature range close to the solidus, or in the annealed state, steel can be classified as follows:
- subeutectoid with ferrite-pearlite structure;
- eutectoid with pearlite structure;
- hypereutectoid containing secondary carbides separated from austenite;
- ledeburite steel, in the structure of which there are primary carbides released during crystallization;
- ferritic or austenitic with precipitation of carbides or intermetallic phases. Typically these are steels with a high content of alloying elements and low carbon content;
- ferritic-martensitic or ferritic-austenitic steel with most often high-temperature ferrite δ.
All grades of alloy steel are divided into three subtypes depending on the amount of useful impurities:
- Low alloy – the percentage of additives is about 2.5%. Adding some positive qualities with virtually unchanged basic characteristics.
- Medium alloyed - the percentage of additives is about 10%. The most commonly used connection.
- Highly alloyed - the percentage of additives varies from 10 to 50%. High-alloy steel is the most durable and expensive.
Regardless of the percentage of additives in the metal composition, steel is divided into 3 subtypes:
- Tooling is a heat-resistant material used in the production of machine and hand tools (drills, cutters, steel cutters, and so on).
- Structural – durable steel that can withstand high dynamic and static loads. It is used in the manufacture of engines and steel mechanisms in mechanical engineering, and is used in the construction and machine tool industries.
- With special properties - steel characterized by chemical and thermal resistance (stainless, acid-resistant, magnetic, wear-resistant, transformer and other types). A number of researchers propose a separate division for this type of steel:
- Heat resistant – able to withstand temperatures up to 1000 degrees.
- Scale-resistant and heat-resistant - steels that are immune to decay.
- Corrosion resistant – used in the production of products operating in high humidity conditions.
Properties and purpose
The most commonly used alloying elements are nickel, manganese, chromium, silicon, lead, selenium and boron. Less commonly used are aluminum, copper, niobium, zirconium and tungsten. The purposes of these elements are very diverse, and when used in the right proportions, steels are obtained with certain characteristics, which, however, cannot be achieved with ordinary carbon steels. Alloys are usually classified based on the elements , the content of which is the highest, and which are called basic components. Elements that are found in smaller proportions are considered secondary components.
Iron itself is not particularly strong, but its strength increases significantly when it is alloyed with carbon and then quickly cooled to produce steel. Some characteristics of steel - soft, semi-soft, semi-hard, hard - are largely determined by the carbon content, which can range from 0.10 to 1.15%.
Risks
Some ferroalloys are produced and used in fine particle form; Airborne dust poses potential toxicity, fire and explosion hazards. In addition, occupational exposure to fumes from the manufacture of certain alloys can lead to serious health problems. A number of tin alloys are hazardous to health (especially at high temperatures) due to the harmful properties of the metals with which tin can be alloyed (for example, lead).
Practical application of alloying additives
Nickel, osmium, ruthenium, copper, gold, silver and iridium are alloyed with platinum to increase hardness. Alloys formed with cobalt have gained importance due to their ferromagnetic properties. Rhodium is used as an anti-corrosion electrolytic coating to protect silver from tarnishing. Rhodium is alloyed with platinum and palladium to make very hard alloys. The purpose of alloying with copper is to increase corrosion resistance. Silver is also alloyed with copper. In its pure form, silver is too soft for coins, cutlery and jewelry; for all applications, it is hardened by alloying with copper.
Ferrous alloys
Ferrous alloys are iron and its alloys. The significant carbon content makes cast iron very brittle. Despite their brittleness and lower mechanical properties than steel, their low cost, ease of casting and specific characteristics make them one of the world's most valuable products with the largest production tonnage.
Non-ferrous alloys
Non-ferrous alloys are alloys that do not contain iron or contain relatively small amounts of iron. Their characteristics are significant corrosion resistance, high electrical and thermal conductivity, low density and ease of production.
Stainless steel
The general characteristics of stainless steel make it a universal material that adapts well to the requirements of today. Any type of alloy has its own advantages depending on the chemical composition.
Aesthetics. There are a number of surface finishes available, from matte to glossy, satin to engraved. Finishes can also be patterned or painted, making stainless steel a unique and aesthetically pleasing material. Architects often choose this material for construction work, interior design and urban furniture.
Mechanical properties: Stainless steel has better mechanical properties at room temperature compared to other materials, which is an advantage in the construction sector as it allows for weight savings per m² or smaller dimensions of structural elements. Good elasticity and hardness combined with good wear resistance (friction, abrasion, impact, elasticity...) allow stainless steel to be used in a wide range of projects. In addition, stainless steel can be installed on a construction site, despite winter temperatures, without the risk of brittleness or breakage, which does not prevent construction time from being extended.
Fire resistance. Compared to other metals, stainless steel has better fire resistance in construction due to its high melting point (above 800 °C). Stainless steel does not emit toxic fumes. Corrosion Resistance: With a chromium content of 10.5%, stainless steel is permanently protected by a passive layer of chromium oxide that forms naturally on its surface when exposed to air humidity. If the surface is damaged, the passive layer is restored. This provides corrosion resistance.
Stamps
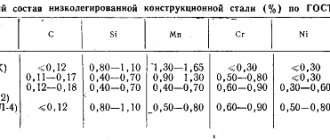
In the CIS, alphanumeric marking of alloy steels is used. The letters indicate the main alloying additives, the numbers following the letters indicate the percentage of their content in the alloy (rounded to the nearest whole number). If the metal contains no more than 1.5% of one or another additive, the number is not given. The percentage of carbon × 100 is indicated at the beginning of the name of the steel. The letter A in the middle of the marking indicates the nitrogen content. If two A's appear at the end, this indicates extra pure steel. The letter Ш at the end denotes particularly high quality steel.
The marking may be supplemented with other designations, for example:
- E - electrical;
- P - high-speed;
- A - automatic;
- L - obtained by casting.
Comprehensive lists of alloy steel grades are specified in GOST 4543-71.
Steel alloying
Alloying of steel is necessary for the manufacture of tools and semiconductors. In the first case, special attention is paid to the mechanical properties, and in the second - to the conductive characteristics. This requires not only different additives (for example, alloying steel with aluminum), but also different technological processes. Alloy steel is an iron-carbon alloy with additional elements (nickel, chromium, molybdenum, cobalt and aluminum) to give it special characteristics such as corrosion resistance, flexibility and hardness, making it superior to ordinary carbon steel.
Alloys are generally designated according to their predominant elements, such as nickel steel, chrome steel and chrome vanadium steel. Alloys can be found in almost all industries, from civil engineering to shipbuilding, oil, automotive and aviation.
The variety of possible alloys is almost endless, as is the variety of characteristics.
Steel alloying
Alloying of steel is necessary for the manufacture of tools and semiconductors. In the first case, special attention is paid to the mechanical properties, and in the second - to the conductive characteristics. This requires not only different additives (for example, alloying steel with aluminum), but also different technological processes. Alloy steel is an iron-carbon alloy with additional elements (nickel, chromium, molybdenum, cobalt and aluminum) to give it special characteristics such as corrosion resistance, flexibility and hardness, making it superior to ordinary carbon steel.
Alloys are generally designated according to their predominant elements, such as nickel steel, chrome steel and chrome vanadium steel. Alloys can be found in almost all industries, from civil engineering to shipbuilding, oil, automotive and aviation.
The variety of possible alloys is almost endless, as is the variety of characteristics.