Steel is one of the most sought after materials in the world today. Without it, it is difficult to imagine any existing construction site, engineering enterprises, and many other places and things that surround us in everyday life. At the same time, this alloy of iron and carbon can be quite different, therefore this article will consider the influence of alloying elements on the properties of steel, as well as its types, grades and purpose.
Classification by purpose
Each steel, depending on what it is created for, can necessarily be classified into one of the following categories:
- Structural.
- Instrumental.
- Special purpose with special properties.
The most numerous class is structural steels, designed to create a variety of building structures, instruments, and machines. Structural grades are divided into upgradeable, cemented, spring-spring, and high-strength.
Tool steels are differentiated depending on the tool for which they are produced: cutting, measuring, etc. It goes without saying that the influence of alloying elements on the properties of steel in this group is also great.
Special steels have their own division, which includes the following groups:
- Stainless (aka corrosion-resistant).
- Heat resistant.
- Heat resistant.
- Electrical.
Use of alloy steel
Today it is almost impossible to name at least one sphere of human activity where there would not be a place for an alloy with such characteristics. Almost all tools are produced from structural and tool steels, for example, milling cutters, cutters, dies, etc. Stainless alloy steels are also used for the production of household products, for example, in the production of utensils and housings for household appliances.
Alloy steel also has many other qualities that guarantee its widest application. It increases the service life of a wide variety of products, ensures their reliability and even allows you to save money. After all, the longer this or that thing is used, the less often you have to purchase a new one.
By the way, products or their components made of alloyed material can be found not only in construction or mechanical engineering, but also in the hands of surgeons, for example, a scalpel, in the production of pipelines. If you make a knife from it, you won’t have to sharpen it often.
Alloy steel products
The scope of use of alloy steels is directly dependent on the heat treatment method to which it has been subjected. Previously, the classification of this material by purpose according to GOST was studied: tool, structural and steel with special qualities.
Low-alloy steels lend themselves well to welding, which is why pipes and other structures are most often made from them. Alloy tool steel is excellent as a raw material for products that will work under pressure.
According to GOST 5950-2000, alloy steel is a material for the production of medical instruments, knives, band saws, etc. This GOST includes all types of its designations and areas of use.
Stainless steel, containing a lot of chromium, is used for the production of pipe products. Pipes made from this material are characterized by increased resistance to rust, and they also perfectly withstand temperature fluctuations, especially high ones.
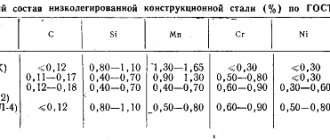
Steel groups by chemical composition
Steels are classified according to the chemical elements that form them:
- Carbon steel grades.
- Alloyed.
Moreover, both of these groups are further divided according to the amount of carbon they contain into:
- Low carbon (carbon less than 0.3%).
- Medium carbon (carbon concentration is 0.3 - 0.7%).
- High carbon (carbon more than 0.7%).
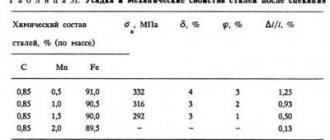
A few words about steel quality
This parameter of a given alloy implies a set of properties, which, in turn, are determined directly by the process of its production. Similar characteristics that alloy tool steels are subject to include:
- Chemical composition.
- Uniformity of structure.
- Manufacturability.
- Mechanical properties.
The quality of any steel directly depends on how much oxygen, hydrogen, nitrogen, sulfur and phosphorus it contains. The method of producing steel also plays an important role. The most accurate method from the point of view of falling into the required range of impurities is the method of steel smelting in electric furnaces.
Properties of alloy steel
Most often, its properties are determined by impurities added during production.
The qualities of steel depend on the alloying elements that are added to its composition:
- rust resistance occurs due to molybdenum and chromium;
- hardness occurs due to manganese, chromium and other components;
- strength is gained through the addition of titanium, manganese, chromium and tungsten.
Alloy steel becomes stronger and more resistant to environmental influences when it contains at least 12% chromium.
Steel, alloyed while maintaining the required percentage of all its elements, will not change its qualities up to a heating temperature of 600 degrees Celsius.
Alloy steel and changes in its properties
Alloy steel, the grades of which contain in their markings the letter designations of forcedly introduced elements, changes its properties not only from these third-party substances, but also from their mutual action with each other.
If we consider carbon specifically, then according to their interaction with it, alloying elements can be divided into two large groups:
- Elements that form a chemical compound (carbide) with carbon are molybdenum, chromium, vanadium, tungsten, manganese.
- Elements that do not create carbides are silicon, aluminum, nickel.
It is worth noting that steels that are alloyed with carbide-forming substances have very high hardness and increased wear resistance.
Low alloy steel (grades: 20KhGS2, 09G2, 12G2SMF, 12KhGN2MFBAYU and others). A special place is occupied by the 13X alloy, which is hard enough to make surgical, engraving, jewelry equipment, and razors from it.
The influence of nickel on the properties of steels
Nickel does not form carbides in steels. In steels, it is an element that promotes the formation and preservation of austenite. Nickel increases the hardening of steels. In combination with chromium and molybdenum, nickel further increases the thermal hardening ability of steels and helps to increase the toughness and fatigue strength of steels. By dissolving in ferrite, nickel increases its viscosity. Nickel increases the corrosion resistance of chromium-nickel austenitic steels in non-oxidizing acid solutions.
Decoding
The content of alloying elements in steel can be determined by its marking. Each of these components introduced into the alloy has its own letter designation. For example:
- Chromium – Cr.
- Vanadium –V.
- Manganese –Mn.
- Niobium – Nb.
- Tungsten –W.
- Titanium – Ti.
Sometimes there are letters at the beginning of the steel grade index. Each of them carries a special meaning. In particular, the letter “P” means that the steel is high-speed, “W” indicates that the steel is ball-bearing, “A” is automatic, “E” is electrical, etc. High-quality steels have a number and letter designation at the end the letter “A”, and especially high-quality ones contain the letter “Ш” at the very end of the marking.
Alloy steel grades
Alloy steel grades vary. They are presented in a wide variety. Depending on the purpose of the steel, its marking is determined.
Today there are a large number of requirements for marking alloy steel. Numerical and alphabetic notations are used for this process. First, numbers are used for marking. They are indicators of how many hundredths of carbon are contained in a particular type of alloy steel. After the numbers there are letters, which indicate which alloying additives were used in the production of a particular type of alloy steel.
The letters may be followed by numbers indicating the amount of alloying substance in the steel material. If there is no digital designation after the designation of any alloying element, then it contains a minimum amount of it, not reaching even one percent.
Table 1. Comparison of steel grades of type Cm and Fe according to international standards ISO 630-80 and ISO 1052-82.
| Steel grades | |||
| St | Fe | St | Fe |
| One hundred | Fe310-0 | St4kp | Fe430-A |
| St1kp | St4ps | Fe430-B | |
| St1ps | St4sp | Fe430-C | |
| St1sp | — | — | Fe430-D |
| St2kp | St5ps | Fe510-B, Fe490 | |
| St2ps | St5Gps | Fe510-B, Fe490 | |
| St2sp | Sg5sp | Fe510-C, Fe490 | |
| StZkp | Fe360-A | ||
| StZps | Fe360-B | St6ps | Fe590 |
| StZGps | Fe360-B | Stbsp | Fe590 |
| StZsp | Fe360-C | Fe690 | |
| StZGsp | Fe360-C | — | |
| Fe360-D | |||
Table 2. Symbols of alloying elements in metals and alloys
| Element | Symbol | Designation of elements in grades of metals and alloys | Element | Symbol | Designation of elements in grades of metals and alloys | ||
| black | colored | black | colored | ||||
| Nitrogen | N | A | — | Neodymium | Nd | — | Nm |
| Aluminum | A1 | YU | A | Nickel | Ni | — | N |
| Barium | Va | — | Br | Niobium | Nb | B | Np |
| Beryllium | Be | L | Tin | Sn | — | ABOUT | |
| Bor | IN | R | — | Osmium | Os | — | OS |
| Vanadia | V | f | To you | Palladium | Pd | — | front |
| bismuth | Bi | In and | In and | Platinum | Pt | — | Pl |
| Tungsten | W | IN | — | Praseodymium | Pr | — | Etc |
| Gadolinium | Gd | — | Gn | Rhenium | Re | — | Re |
| Gallium | Ga | Gi | Gi | Rhodium | Rh | — | Rg |
| Hafnia | Hf | — | Gf | Mercury | Hg | — | R |
| Germanium | Ge | — | G | Ruthenium | Ru | — | Pv |
| Holmium | But | — | GOM | Samarium | Sm | — | Myself |
| Dysprosium | Dv | — | DIM | Lead | Pb | — | WITH |
| Europium | Eu | — | Ev | Selenium | Se | TO | ST |
| Iron | Fe | — | AND | Silver | Ag | — | Wed |
| Gold | Au | — | Evil | Scandium | Sc | — | From km |
| Indium | In | — | In | Antimony | Sb | — | Cv |
| Iridium | Ir | — | AND | Thallium | Tl | — | Tl |
| Ytterbium | Yb | — | ITN | Tantalum | Ta | — | TT |
| Yttrium | Y | — | THEM | Tellurium | Those | — | T |
| Cadmium | Cd | CD | CD | Terbium | Tb | — | Volume |
| Cobalt | Co | TO | TO | Titanium | Ti | T | TPD |
| Silicon | Si | WITH | Kr(K) | T\'liy | Tm | — | TUM |
| Lanthanum | La | — | La | Carbon | WITH | U | — |
| Lithium | Li | — | Le | Phosphorus | P | P | F |
| Lutetium | Lu | — | Leung | Chromium | Cr | X | X(Xp) |
| Magnesium | Mg | Sh | Mg | Cerium | Ce | — | Xie |
| Manganese | Mn | G | Mts(Mr) | Zinc | Zn | — | C |
| Copper | Cu | D | M | Zirconium | Zr | C | TsEV |
| Molybdenum | Mo | M | — | Erbium | Er | — | Erm |
Impact of alloying elements
First of all, it should be said that carbon has a fundamental influence on the properties of steel. It is this element that, with increasing concentration, provides an increase in strength and hardness while reducing viscosity and plasticity. In addition, increased carbon concentration guarantees deterioration in machinability.
The chromium content of steel directly affects its corrosion resistance. This chemical element forms a thin protective oxide film on the surface of the alloy in an aggressive oxidizing environment. However, to achieve this effect, the steel must contain at least 11.7% chromium.
Aluminum deserves special attention. It is used in the process of alloying steel to remove oxygen and nitrogen after purging it in order to help reduce the aging of the alloy. In addition, aluminum significantly increases impact strength and fluidity, and neutralizes the extremely harmful effects of phosphorus.
Vanadium is a special alloying element that gives alloyed tool steels high hardness and strength. At the same time, the grain in the alloy decreases and the density increases.
Alloy steel, the grades of which contain tungsten, is endowed with high hardness and red resistance. Tungsten is also good because it completely eliminates brittleness during the planned tempering of the alloy.
To increase heat resistance, magnetic properties and resistance to significant impact loads, steel is alloyed with cobalt. But one of those elements that does not have any significant effect on steel is silicon. However, in those grades of steel that are intended for welded metal structures, the silicon concentration must necessarily be in the range of 0.12-0.25%.
Magnesium significantly increases the mechanical properties of steel. It is also used as a desulfurizer in the case of off-furnace desulfurization of cast iron.
Low-alloy steel (its grades contain alloying elements less than 2.5%) very often contains manganese, which provides it with an inevitable increase in hardness and wear resistance while maintaining optimal ductility. But the concentration of this element must be more than 1%, otherwise it will not be possible to achieve the specified properties.
Carbon steel grades, smelted for various large-scale building structures, contain copper, which provides maximum anti-corrosion properties.
To increase red-hardness, elasticity, tensile strength and corrosion resistance, molybdenum is necessarily introduced into the steel, which also increases the resistance to oxidation of the metal when heated to high temperatures. In turn, cerium and neodymium are used to reduce the porosity of the alloy.
When considering the influence of alloying elements on the properties of steel, one cannot ignore nickel. This metal allows steel to achieve excellent hardenability and strength, increase ductility and impact resistance, and lower the cold brittleness limit.
Niobium is also widely used as an alloying additive. Its concentration, 6-10 times higher than the amount of carbon necessarily present in the alloy, eliminates intergranular corrosion of stainless steel and protects welds from extremely unwanted destruction.
Titanium allows you to obtain the most optimal strength and ductility indicators, as well as improve corrosion resistance. Those steels that contain this additive are very well processed with various special-purpose tools on modern metal-cutting machines.
The introduction of zirconium into a steel alloy makes it possible to obtain the required grain size and, if necessary, influence grain growth.
The influence of molybdenum on the properties of steels
Molybdenum readily forms carbides in steels. It dissolves only slightly in cementite. Molybdenum forms molybdenum carbides once the carbon content of the steel becomes high enough. Molybdenum is capable of providing additional thermal hardening during tempering of hardened steels. It increases the creep resistance of low-alloy steels at high temperatures.
Molybdenum additives help refine the grain of steels, increase the hardening of steels by heat treatment, and increase the fatigue strength of steels. Alloy steels containing 0.20-0.40% molybdenum or the same amount of vanadium slow down the occurrence of temper brittleness, but do not completely eliminate it. Molybdenum improves the corrosion resistance of steels and is therefore widely used in high-alloy ferritic stainless steels and in chromium-nickel austenitic stainless steels. High molybdenum content reduces the susceptibility of stainless steel to pitting corrosion. Molybdenum has a very strong solid solution strengthening effect on austenitic steels that are used at elevated temperatures.