Change in structure upon addition of carbon
Indicators of strength and ductility depend on the structure and its changes with increasing carbon content.
At a proportion of up to 0.2%, ferrite and tertiary cementite are formed; a further increase leads to the formation of eutectoid ferrite and cementite (pearlite). The perlite index value gradually increases and at 0.8% carbon only perlite is contained. If the content is more than 0.8%, needles of secondary cementite and perlite appear.
The formation of cementite occurs up to 2% carbon, while the strength decreases due to the fragility of the cementite network along the boundaries of pearlite grains. When this value is exceeded, a eutectic mixture is formed.
What does the carbohydrate contained in steel provide?
The amount of cementite will increase as the carbon content of steel increases. In this case, the proportion of ferrite will simultaneously decrease. If the ratio between the components is changed, the ductility will decrease, and the strength and hardness will increase. The strength will increase as long as the carbon content is 1%, but after that it will certainly decrease because a cementite coarse network will be formed.
In simple Russian terms, carbon has a direct effect on viscosity properties. If the amount of carbon in the alloy is increased, the product will not succumb to sudden brittleness, and the impact strength will decrease.
In addition, there are other processes that can cause an increase in carbon composition:
- — electrical resistance will increase;
- — coercive force increases;
- — the permeability of magnets will be reduced;
- - the induction of magnets will become less dense.
In addition, we must remember that carbon can also affect technological processes. In addition to all the positive aspects described above, the casting properties of steel will be significantly deteriorated as soon as the carbon content in the composition increases. Moreover, weldability will be significantly worse and cutting and forming such steels will be much more difficult. But this does not mean that if the steel does not contain carbon, then no problems will arise with it. Steels with a low carbon content will also be difficult to cut.
But, in addition to carbon, steel may contain other impurities, which must also be kept in mind. Such impurities are divided into three permanent groups:
1. Standard. These include silicon, sulfur, phosphorus, manganese. In this case, the first and last are considered technological type impurities. These impurities are introduced during the steel smelting process itself to deoxidize it.
2. Hidden. This includes gases such as oxygen, hydrogen, nitrogen. They will enter the steel directly during smelting. Thanks to them, the resistance to brittle fracture will be reduced.
New properties and advantages of the alloy
Carbon in steel gives it additional advantages, primarily:
- sufficient hardness of the surface layer and relative softness of the inner layer;
- good machinability;
- durability;
- affordable price.
With an increase in the proportion of carbon, hardness and strength increase and ductility decreases, therefore, the more carbon there is, the more difficult the cutting process, and the worse the deformation and welding performance. Based on this, the following types of steel are distinguished:
- Low carbon, with a share of less than 0.25%. They are quite plastic, easy to deform and process.
- Medium carbon, with a share of 0.3-0.6%. This type is also plastic and has an average strength rating.
- High carbon, with a share of 0.6-2%. With low viscosity and high strength. Welding is carried out only with preheating to 225 degrees.
In addition to the basic mechanical properties, an increase in carbon content increases the cold brittleness threshold.
The essence of the steel improvement process
After hardening of steel, martensite structures predominate in it. High tempering of steel consists of heating at least 20-40°C below the Ac1 point (see Iron-carbon diagram), but not lower than 500°C, holding and controlled cooling of the part.
Improvement of steels on the iron-carbon diagram
At the second stage of steel improvement - the process of high tempering of steel - diffusion decomposition of martensite occurs until the formation of tempered sorbitol (see Elements of the theory of heat treatment). Dispensed sorbitol has a homogeneous and dispersed structure.
Application of carbon steel
Applications depend on the mechanical properties, and therefore on how much carbon there is in the steel. With an index of 0.7-1.3%, carbon steel is used for the manufacture of cutting and impact tools. They are marked with the letter “U”, the subsequent number characterizes the share, for example, U13. The higher the indicator, the greater the influence of carbon on the mechanical properties of steel.
Low-carbon steels are divided into subgroups depending on their purpose:
- Low carbon: 05, 08, 10. Due to their plasticity, they are used in cold stamping for the manufacture of washers, gaskets, casings and other parts.
- Low-carbon: 15, 20, 25. This value of carbon in the steel composition gives increased hardness and sufficient toughness; they are used for the manufacture of small-sized parts (cams, pushers, lightly loaded gears).
- Medium carbon: 30, 35, 40, 45, 50, 55. Used for the manufacture of crankshafts of low-speed engines, gears, flywheels - parts whose performance is determined by fatigue resistance. They are used after normalization and surface hardening, which increase viscosity and ductility, and accordingly, the machinability index improves.
- High carbon: 60, 65, 70, 75, 80, 85. Used for the manufacture of springs, eccentrics and springs. They are pre-hardened and moderately tempered, which improves the elastic properties necessary for manufactured parts.
- Boiler rooms: 12K-22K. Used for the manufacture of equipment operated at high temperatures (vessels and boilers for turbines and combustion chambers).
- Automatic steel. It has found application for the manufacture of automotive fasteners under static loads (studs, nuts, bolts).
Materials scientist
Steels are the most common materials. They have good technological properties. Products are obtained as a result of pressure and cutting processing.
The advantage is the ability to obtain the desired set of properties by changing the composition and type of processing. Steels are divided into carbon and alloy.
The influence of carbon and impurities on the properties of steels
Carbon steels are the main ones. Their properties are determined by the amount of carbon and the content of impurities that interact with iron and carbon.
Carbon influence.
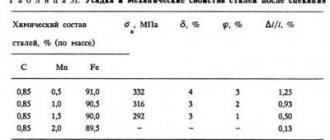
The effect of carbon on the properties of steels is shown in Fig. 10.1
Fig. 10.1. The influence of carbon on the properties of steels
As the carbon content in the steel structure increases, the amount of cementite increases, while the proportion of ferrite decreases. Changing the ratio between the components leads to a decrease in ductility, as well as an increase in strength and hardness. Strength increases up to a carbon content of about 1%, and then it decreases as a coarse network of secondary cementite is formed.
Carbon affects viscous properties. Increasing the carbon content increases the threshold of cold brittleness and reduces toughness.
Electrical resistance and coercive force increase, magnetic permeability and magnetic induction density decrease.
Carbon also affects technological properties. An increase in carbon content worsens the casting properties of steel (steels with a carbon content of up to 0.4% are used), workability by pressure and cutting, and weldability. It should be taken into account that steels with low carbon content are also difficult to cut.
Impact of impurities.
Steels always contain impurities, which are divided into four groups. 1. Permanent impurities: silicon, manganese, sulfur, phosphorus.
Manganese and silicon are introduced during the steelmaking process for deoxidation; they are technological impurities.
The manganese content does not exceed 0.5...0.8%. Manganese increases strength without reducing ductility, and sharply reduces the red brittleness of steel caused by the influence of sulfur. It helps reduce the content of iron sulfide FeS, as it forms a compound with sulfur, manganese sulfide MnS. Manganese sulfide particles are located in the form of separate inclusions, which are deformed and appear elongated along the rolling direction.
The silicon content does not exceed 0.35...0.4%. Silicon, by degassing the metal, increases the density of the ingot. Silicon dissolves in ferrite and increases the strength of steel, especially the yield strength increases. But there is a slight decrease in ductility, which reduces the ability of steel to draw
The phosphorus content in steel is 0.025...0.045%. Phosphorus, dissolving in ferrite, distorts the crystal lattice and increases the tensile strength and yield strength, but reduces ductility and toughness.
Being located near the grains, it increases the temperature of transition to a brittle state, causes cold brittleness, reduces the work of crack propagation. An increase in phosphorus content by every 0.01% increases the threshold of cold brittleness by 20...25ºС.
Phosphorus has a tendency to segregate, so in the center of the ingot, individual areas have a sharply reduced viscosity.
For some steels, it is possible to increase the phosphorus content to 0.10...0.15% to improve machinability.
Sulfur reduces ductility, impairs weldability and corrosion resistance.
The sulfur content in steels is 0.025...0.06%. Sulfur is a harmful impurity that gets into steel from cast iron. When interacting with iron, it forms a chemical compound - sulfur sulfide FeS, which, in turn, forms a low-melting eutectic with iron with a melting point of 988ºC. When heated for rolling or forging, the eutectic melts and the bonds between the grains are broken. During deformation, tears and cracks appear at the locations of the eutectic, and the workpiece is destroyed—the phenomenon of red brittleness.
Red brittleness – increased brittleness at high temperatures
Sulfur reduces mechanical properties, especially impact strength and ductility (and), as well as endurance limit. It impairs weldability and corrosion resistance.
2. Hidden impurities - gases (nitrogen, oxygen, hydrogen) - enter the steel during smelting.
Nitrogen and oxygen are found in steel in the form of brittle non-metallic inclusions: oxides (FeO, SiO2, Al2O3) nitrides (Fe 2N), in the form of a solid solution or in a free state, located in defects (cavities, cracks).
Interstitial impurities (nitrogen N, oxygen O) increase the threshold of cold brittleness and reduce the resistance to brittle fracture. Non-metallic inclusions (oxides, nitrides), being stress concentrators, can significantly reduce the endurance limit and viscosity.
Hydrogen dissolved in steel is very harmful, as it significantly embrittles the steel. It leads to the formation of flakes in rolled billets and forgings.
Flocks are thin cracks of an oval or round shape, having the appearance of spots at the fracture - silvery flakes.
Metal with flakes cannot be used in industry; during welding, cold cracks form in the deposited and base metal.
If hydrogen is in the surface layer, then it is removed as a result of heating at 150...180, preferably in a vacuum, mmHg. Art.
Vacuuming is used to remove hidden impurities.
3. Special impurities that are specially introduced into steel to obtain specified properties. Impurities are called alloying elements, and steels are called alloyed steels.
Purpose of alloying elements.
The main alloying element is chromium (0.8…1.2)%. It increases hardenability and helps to obtain high and uniform hardness of steel. The cold brittleness threshold of chromium steels is (0…-100) ºС.
Additional alloying elements.
Boron - 0.003%. Increases hardenability and also increases the threshold of cold brittleness (+20…-60) ºС.
Manganese – increases hardenability, but promotes grain growth and increases the threshold of cold brittleness to (+40…-60) ºС.
Titanium (~0.1%) is introduced to refine the grain in chromium-manganese steel.
The introduction of molybdenum (0.15...0.46%) into chromium steels increases hardenability and lowers the cold brittleness threshold to -20...-120 ºС. Molybdenum increases the static, dynamic and fatigue strength of steel and eliminates the tendency to internal oxidation. In addition, molybdenum reduces the tendency of steels containing nickel to become temper brittle.
Vanadium in an amount of (0.1...0.3)% in chromium steels refines the grain and increases strength and toughness.
The introduction of nickel into chromium steels significantly increases strength and hardenability, lowers the threshold of cold brittleness, but at the same time increases the tendency to temper brittleness (this disadvantage is compensated by the introduction of molybdenum into the steel). Chromium-nickel steels have the best range of properties. However, nickel is scarce and the use of such steels is limited.
A significant amount of nickel can be replaced with copper, this does not lead to a decrease in viscosity.
When chromium-manganese steels are alloyed with silicon, steels are obtained - chromansil (20KhGS, 30KhGSA). Steels have a good combination of strength and toughness, are well welded, stamped and machined. Silicon increases impact strength and temperature reserve of viscosity.
The addition of lead and calcium improves machinability. The use of hardening heat treatment improves the complex of mechanical properties.
Distribution of alloying elements in steel.
Alloying elements dissolve in the main phases of iron-carbon alloys (ferrite, austenite, cementite), or form special carbides.
The dissolution of alloying elements occurs as a result of the replacement of iron atoms with atoms of these elements. These atoms create stresses in the lattice, which cause a change in its period.
Changing the dimensions of the lattice causes a change in the properties of ferrite - strength increases, ductility decreases. Chromium, molybdenum and tungsten strengthen less than nickel, silicon and manganese. Molybdenum and tungsten, as well as silicon and manganese in certain quantities, reduce viscosity.
In steels, carbides are formed by metals located in the periodic table to the left of iron (chromium, vanadium, titanium), which have a less complete d-electron band.
In the process of carbide formation, carbon donates its valence electrons to fill the d electron band of the metal atom, while in the metal the valence electrons form a metallic bond, which determines the metallic properties of carbides.
When the ratio of the atomic radii of carbon and metal is more than 0.59, typical chemical compounds are formed: Fe3C, Mn3C, Cr23C6, Cr7C3, Fe3W3C - which have a complex crystal lattice and dissolve in austenite when heated.
When the ratio of the atomic radii of carbon and metal is less than 0.59, interstitial phases are formed: Mo2C, WC, VC, TiC, TaC, W2C - which have a simple crystal lattice and are difficult to dissolve in austenite.
All carbides have high hardness and melting point.
4. Random impurities.
Classification and marking of steels
Steel classification
Steels are classified according to many characteristics.
- By chemical composition: carbon and alloyed.
- By carbon content:
a) low-carbon, with a carbon content of up to 0.25%; b) medium-carbon, with a carbon content of 0.3...0.6%; c) high-carbon, with a carbon content above 0.7%
- According to the equilibrium structure: hypoeutectoid, eutectoid, hypereutectoid.
- By quality. A quantitative indicator of quality is the content of harmful impurities: sulfur and phosphorus:
a) carbon steels of ordinary quality: b) high-quality steels; c) high-quality steels.
- By smelting method:
a) in open hearth furnaces; b) in oxygen converters; c) in electric furnaces: electric arc, induction, etc.
- By purpose:
a) structural – used for the manufacture of machine parts and mechanisms; b) instrumental – used for the manufacture of various tools; c) special – steels with special properties: electrical, with special magnetic properties, etc.
Steel marking
Alphanumeric designation of steels has been adopted
Carbon steels of ordinary quality (GOST 380).
Steels contain increased amounts of sulfur and phosphorus
Marked: St.2kp., BSt.3kp, VSt.3ps, VSt.4sp.
St – index of this steel group. The numbers from 0 to 6 are the conventional number of the steel grade. As the grade number increases, the strength of the steel increases and the ductility decreases. According to guarantees upon delivery, there are three groups of steels: A, B and C. For steels of group A, mechanical properties are guaranteed upon delivery; the index of group A is not indicated in the designation. For steels of group B, the chemical composition is guaranteed. For group B steels, both mechanical properties and chemical composition are guaranteed upon delivery.
The indices kp, ps, sp indicate the degree of deoxidation of the steel: kp - boiling, ps - semi-calm, sp - calm.
Quality carbon steels
High-quality steels are supplied with guaranteed mechanical properties and chemical composition (group B). The degree of deoxidation is generally calm.
Structural quality carbon steels. They are marked with a two-digit number indicating the average carbon content in hundredths of a percent. The degree of deoxidation is indicated if it differs from calm.
Steel 08 kp, steel 10 ps, steel 45.
The carbon content is, respectively, 0.08%, 0.10%, 0.45%.
High-quality tool carbon steels are marked with the letter U (carbon tool steel) and a number indicating the carbon content in tenths of a percent.
Steel U8, steel U13.
Carbon content 0.8% and 1.3% respectively
Tool high-quality carbon steels. They are marked similarly to high-quality tool carbon steels, only at the end of the mark they put the letter A to indicate the high quality of the steel.
Steel U10A.
Quality and high quality alloy steels
Designation is alphanumeric. Alloying elements have symbols, designated by letters of the Russian alphabet.
Designations of alloying elements:
X – chromium, N – nickel, M – molybdenum, B – tungsten, K – cobalt, T – titanium, A – nitrogen (indicated in the middle of the mark), G – manganese, D – copper, F – vanadium, C – silicon, P – phosphorus, P – boron, B – niobium, C – zirconium, Y – aluminum.
Alloy structural steels
Steel 15Х25Н19ВС2
At the beginning of the stamp there is a two-digit number indicating the carbon content in hundredths of a percent. Alloying elements are listed below. The number following the symbol of the element shows its content as a percentage,
If the number does not appear, then the content of the element does not exceed 1.5%.
The specified grade of steel contains 0.15% carbon, 35% chromium, 19% nickel, up to 1.5% tungsten, up to 2% silicon.
To designate high-quality alloy steels, the symbol A is indicated at the end of the grade.
Alloy tool steels
Steel 9ХС, steel ХВГ.
At the beginning of the brand there is a single-digit number indicating the carbon content in tenths of a percent. If the carbon content is more than 1%, the number is not indicated,
Alloying elements are listed below, indicating their content.
All alloy tool steels are high quality.
Some steels have non-standard designations.
High-speed tool steels
Steel P18
P – index of this group of steels (from rapid – speed). Carbon content more than 1%. The number shows the content of the main alloying element - tungsten.
The tungsten content in this steel is 18%.
If steels contain an alloying element, then their content is indicated after the designation of the corresponding element.
Ball bearing steels
Steel ШХ6, steel ШХ15ГС
Ш – index of this group of steels. X - indicates the presence of chromium in the steel. The following number shows the chromium content in tenths of a percent; in the indicated steels, 0.6% and 1.5%, respectively. Alloying elements included in the steel composition are also indicated. Carbon content more than 1%.
Influence of other impurities
Like carbon, other chemical elements in steel affect its mechanical properties:
- silicon – used as an active deoxidizer;
- manganese – reduces the influence of oxygen and sulfur, reduces resistance to stress;
- sulfur and phosphorus – increase the red brittleness index and belong to the category of harmful impurities;
- titanium – improves strength and ductility;
- chromium – increases heat resistance and abrasion resistance;
- nickel – improves viscosity and elasticity;
- copper – affects corrosion resistance.
The mechanical properties of steel depend entirely on its composition and the presence of certain impurities. It is these characteristics that must be taken into account when using steel in industrial production. Some negative effects of element content can be reduced by additional improvement methods - thermal hardening of the surface (cementation) or the addition of anti-corrosion protection, in other words - galvanization, the coating of which increases the service life of the product.
Steel classification
Steels are classified according to their intended purpose for further use, chemical composition, quality, and structure.
According to their purpose, steels are usually divided into structural, corrosion-resistant (stainless), instrumental, heat-resistant, and cryogenic.
- Alloyed - steel containing specially introduced, in certain quantities, elements that provide the required physical or mechanical properties. These elements are called alloying elements. As a rule, alloying increases the strength and corrosion resistance of steel and reduces brittleness. Alloy steel according to the degree of alloying is divided into: low-alloy (alloying elements up to 2.5%); medium alloyed (from 2.5 to 10%); highly alloyed (from 10 to 50%).
- Structural steel is used in the manufacture of various parts, mechanisms and structures in mechanical engineering and construction, and has certain mechanical, physical and chemical properties.
- Stainless steel is an alloy steel that is resistant to corrosion in the atmosphere and aggressive environments.
- Tool carbon - steel with a carbon content of 0.7% and higher. It is characterized by high hardness and strength and is used for making tools.
- Heat-resistant steel is a type of steel that can be used at high temperatures (from 30% of the melting point).
Chromium-nickel-molybdenum-vanadium steels
In addition to molybdenum, vanadium is added, which helps to obtain a fine-grained structure. Steel grades 38KhN3MF and 36Kh2N2MFA are used for parts with large sections (1000...1500 mm and more). After quenching, bainite is formed in the core, and sorbitol is formed after tempering. Steels have high strength, ductility and toughness, and a low cold brittleness threshold. Molybdenum present in steel increases its heat resistance. These steels can be used at temperatures of 400...450 0C in the manufacture of the most critical parts of turbines and compressors, which require material of special strength in large sections (forgings of shafts and solid forged turbine rotors, shafts of high-stress turbo-blowing machines, gearbox parts, etc.).
Source
Chromium-manganese steels
Joint alloying of steels with chromium (0.9...1.2%) and manganese (0.9...1.2%) makes it possible to obtain sufficiently high strength and hardenability (for example, 40KhG), however, they have reduced viscosity, a lower threshold of cold brittleness (from 20 0С to minus 60 0С). The introduction of titanium reduces the tendency to overheat, and the addition of boron increases hardenability.
Table 10 - Mechanical properties of some alloyed steels that can be improved
| steel grade | Calcined diameter, mm | sigmaв, MPa | sigma0.2, MPa | d, % | y, % | KCU, MJ/m2 |
| 30X 40X 40XFA 40ХГТР 30ХГС 40ХН 30ХН3А 40ХН2МА 36Х2Н2МФА 38ХН3МФА | 25-35 25-35 25-35 50-75 50-75 50-75 75-100 75-100 more than 100 more than 100 | 900 1000 900 1000 1100 1000 1000 1100 1200 1200 | 700 800 750 800 850 800 800 950 1100 1100 | 12 10 10 11 10 11 10 12 12 12 | 45 45 50 45 45 45 50 50 50 50 | 0,7 0,6 0,9 0,8 0,4 0,7 0,8 0,8 0,8 0,8 |