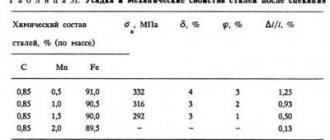
The influence of carbon on the properties of steels
Carbon is the main strengthening element in all steels except austenitic stainless steels and some other high-alloy steels. The strengthening effect of carbon consists of solid solution strengthening and strengthening due to dispersed precipitation of carbides. With increasing carbon content in steel, its strength increases, but ductility and weldability decreases.
Carbon has a moderate tendency to macrosegregate during crystallization. Macrosegregation of carbon is usually more significant than that of all other alloying elements. Carbon has a strong tendency to segregate at defects in steels such as grain boundaries and dislocations. Carbide-forming elements can react with carbon to form “alloyed” carbides.
Manganese steel grades
- LGM
- Casting
- Laboratory
- Stumping
- Heat treatment
- Mechanical restoration
- Video
- Products Steel castings Drawings (Steel)
- Drawings (Manganese steel)
- Manganese steel
- Heat resistant steel
- Casting steel
- Structural alloy steel
- Alloy steel
- Carbon steel
- Cast Iron Drawings (Cast Iron)
- Cast iron
- Chrome cast iron
- Pig iron
- Tubing
- Cast iron ring weights (UChK)
- Housings
- Art casting Art casting
- Park casting
- LGM
- Casting
- Laboratory
- Stumping
- Heat treatment
- Mechanical restoration
- Video
- Steel casting Drawings (Steel)
Drawings (Manganese steel)
- Cast Iron Drawings (Cast Iron)
- Cast iron
- Chrome cast iron
- Pig iron
- Tubing
- Cast iron ring weights (UChK)
- Housings
- Art casting Art casting
- Park casting
The influence of manganese on the properties of steels
Manganese is present in almost all steels in amounts of 0.30% or more. Manganese is used to remove oxygen and sulfur from steel. It has less tendency to segregate than any other alloying element. Manganese has a beneficial effect on surface quality over the entire carbon content range, with the exception of very low carbon steels, and also reduces the risk of red brittleness. Manganese has a beneficial effect on the ductility and weldability of steels.
Manganese does not form its own carbide, but only dissolves in cementite and forms alloyed cementite in steels. Manganese promotes the formation of austenite and therefore expands the austenite region of the phase diagram. High manganese content (more than 2%) leads to an increased tendency to cracking and warping during hardening. The presence of manganese in steels encourages impurities such as phosphorus, tin, antimony and arsenic to segregate to the grain boundaries, causing temper brittleness.
Manganese steel - grade
Low-carbon manganese steels of grades 10G2A and 12G2A have high ductility and good weldability. They are used for the manufacture of stamp-welded parts. [1]
When processing manganese steel grade G12 (manganese content 12–94%), it was found that the most suitable for such processing is a cutter made of hard alloy grade T15K6, which has sufficient durability at a cutting speed of 13–6 m/min. The T5KYu alloy provides satisfactory durability (50 min. However, the T15K6 alloy is relatively brittle and does not work well under impact loads. [2]
Crankshaft 3 is made of manganese steel grade 50G and rests on five main bearings. The surfaces of the shaft journals are hardened by high frequency currents. The diameter of the main neck is 88-9 mm, the diameter of the crank is 70 mm. To balance the centrifugal forces, counterweights are installed on the first and fourth cranks of the shaft. [3]
Steel with high wear resistance is manganese steel grade G13, containing 1 0 - 1 3% C; 11 0 - 14 0% MP. It belongs to the austenitic class. [4]
For welding main pipelines, wire made of carbon steel grades SV-08 and SV-08A and manganese steel grades SV-08g-A is used. The letter A in the wire grade means that the wire contains significantly less harmful impurities - sulfur and phosphorus, therefore such wire is used for more demanding work. [5]
The regenerator consists of housing 1, the lower part of which (up to the flange connection) is made of chromium-nickel steel grade X18N9T, and the upper part is made of manganese steel grade 09G2DT / m; 2 coils made of copper or steel tubes, and 3 stone nozzles with a granule size of 4 - 10 mm. The coils rest on the bottom of the housing. The coil collectors are routed through seals 4 and 5, located in the bottom and cover. Air is introduced into the regenerator and the return flow is output through a perforated cone 6, covered with a stainless steel mesh 7, and the air is removed and the return flow is introduced through an annular perforated manifold 8, also covered with a stainless steel mesh. [6]
Alloy steels and iron-based alloys with special properties contain a large number of alloying components, the combination of which gives the steels heat resistance, anti-corrosion, high electrical resistance and other valuable properties. For example, steel grade 1Х18Н9Т - chromium-nickel stainless steel containing about 0.1% carbon, 18% chromium, 9% nickel, about 1% titanium, is highly acid-resistant and is used for the manufacture of devices in chemical engineering factories; manganese steel grade PZ, called Hadfield steel, containing from 11 to 14% manganese, works well against abrasion and is used for the manufacture of bucket teeth for excavators and railway switches. [7]
Alloy steels and iron-based alloys with special properties contain a large number of alloying components, the combination of which gives the steels heat resistance, anti-corrosion, high electrical resistance and other valuable properties. For example, steel grade 1Х18Н9Т - chromium-nickel stainless steel containing about 0 1% carbon, 18% chromium, 9% nickel, about 1% titanium, is highly acid-resistant and is used for the manufacture of devices in chemical engineering factories; manganese steel grade G13, called Hadfield steel, containing from 11 to 14% manganese, works well against abrasion and is used for the manufacture of bucket teeth for excavators and railway switches. [8]
In bins designed to store solid lump materials, the inner surface of the inclined walls is lined to protect the walls from abrasion and dents due to impact. The type of lining depends on the abrasive properties of the bulk material. Thus, bunkers for ore and scrap are lined with sheet manganese steel grade ZOG2 with a thickness of 6 - 10 mm. Sometimes wooden lining is used. [10]
The influence of silicon on the properties of steels
Silicon is one of the main deoxidizing agents used in steel smelting. Therefore, the silicon content determines the type of steel produced. Mild carbon steels can contain silicon up to a maximum of 0.60%. Semi-quiet steels may contain moderate amounts of silicon, for example 0.10%.
Silicon is completely dissolved in ferrite at a silicon content of up to 0.30%. It increases the strength of ferrite without almost reducing its ductility. When the silicon content is above 0.40% in general purpose carbon steel, a significant decrease in ductility occurs.
In combination with manganese or molybdenum, silicon provides higher hardenability of steel. The addition of silicon to chromium-nickel austenitic steels increases their resistance to stress corrosion. In thermally hardenable steels, silicon is an important alloying element; it increases the ability of steels to be thermally hardened and their wear resistance, increases the elastic limit and yield strength. Silicon does not form carbides and does not contain cementite or other carbides. It dissolves in martensite and slows down the decomposition of alloyed martensite up to 300 °C.
Deoxidation, degassing and alloying of steel
STEEL ALLOYING
INFLUENCE OF ALLOYING IMPURITIES ON STEEL PROPERTIES
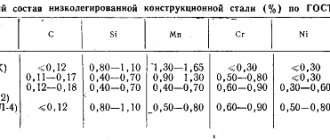
According to the chemical composition, steels can be divided into carbon and alloy. Steel is called alloyed if its given composition determines the content of elements that are absent in ordinary carbon steel in significant quantities, or if there is an increased content of silicon and manganese compared to what is allowed in carbon steel.
The composition of ordinary carbon steel includes the following elements: C, Si, Mn, Al, S, P, O, H and N. The carbon content determines the grade and properties of carbon steel. Silicon, manganese and aluminum are introduced into this steel in small quantities, mainly to deoxidize it. Manganese and silicon also provide the specified mechanical properties of steel. The remaining listed elements enter the finished steel from charge materials or furnace gases and are harmful impurities. In addition to the indicated elements, carbon steel always contains small amounts of chromium, nickel, copper and molybdenum introduced by the charge. In carbon steel smelted at machine-building plants or plants that produce large quantities of alloy steel, the content of these elements is higher.
The most common alloying elements are: Cr, Mn, Ni, Si, W, Mo, V, Ti, Cu, Co, Al, B, Nb, Zr, N, As, S, P.
In modern practice, steel is especially often alloyed with the first eight elements.
The influence of various elements on the properties of steel is summarized below.
Carbon is contained in steel of all grades in an amount from 0.02 to 1.5%. With increasing carbon content, the hardness and strength of steel increase and the ductility of steel decreases. An increase in the amount of carbon by every 0.1% up to 0.85% increases the yield strength by 2.8 kg/mm2, the tensile strength by 6.5 kg/mm2, reduces elongation by 4.3% and cross-sectional compression - by 7.3%. When the carbon content increases to more than 0.85%, its effect on the mechanical properties of steel appears to a lesser extent. Carbon increases the cutting ability of steel, increases electrical resistance, coercive force, and slightly reduces the density of steel; reduces the melting point of steel by approximately 90 ° C for every percentage of carbon. Under the conditions of the steelmaking process, it is a deoxidizing agent and determines the content of oxygen dissolved in liquid steel. In solid steel, carbon forms various structural components with iron, which determines the properties of the steel and is the basis for its subsequent heat treatment.
Manganese is found in all types of steel and is a deoxidizer or alloying element. Manganese in the form of ferromanganese is widely used in steelmaking processes. It facilitates hot processing of steel by pressure, forming refractory compounds with sulfur and oxygen. The residual amount of manganese (0.25-1.0%), dissolving in ferrite and partially forming carbide, has a positive effect on the mechanical properties of steel. Within these limits, manganese improves the hardenability of steel, increases the yield strength of the metal and has almost no effect on elongation. Up to 1.8% Μη is introduced into structural alloy steels. Manganese is an austenite-forming element. High-carbon steel with 13% Μη has an austenitic structure in the hardened state and has good abrasion resistance under impact loads. In combination with tungsten and molybdenum, manganese serves as a substitute for nickel in structural steels, and with nitrogen in stainless steels.
Silicon, which is a stronger deoxidizer than manganese, is introduced into steel for deoxidation in small quantities (0.2-0.4%). With a content of more than 0.8%, silicon is an alloying element. In an amount of about 1%, silicon increases the tensile strength and yield strength of steel without reducing the viscosity of the metal, therefore carbon steel with the specified silicon content is used for the manufacture of springs and springs. Silicon-manganese steels containing silicon and manganese in the range of 1-1.3% have good plastic and strength properties and serve as substitutes for chromium-nickel steel. Silicon increases magnetic permeability and electrical resistance, reduces hysteresis losses, which is why electrical steels contain silicon (1.5-2% in dynamite steel, up to 4% in transformer steel). Silicon, being a ferrite-forming element, increases the acid resistance of the metal. An alloy containing up to 14% silicon (thermosilide) is used for acid-resistant casting.
Aluminum is a powerful deoxidizer. To deoxidize and regulate the size of the primary austenite grain, no more than 0.2% Al is usually introduced into the steel. Aluminum prevents the aging of steel and increases its plastic properties. In chrome-molybdenum and chromium steels intended for nitriding, 0.7-1.2% Al is introduced.
Sulfur in conventional steel grades is contained in an amount of 0.01 - 0.05% and is almost entirely in the form of non-metallic inclusions. It causes red brittleness of steel, reduces mechanical properties, increases the susceptibility of steel to rusting and abrasion, and reduces the ability of steel to deep drawing (stamping). At a higher content, sulfur facilitates the machinability of steel on machines, so 0.1–0.3% S is introduced into special grades of steel (automatic steel).
Phosphorus in steel is usually present in an amount of 0.02-0.1%. It causes cold brittleness in steel. In medium and high carbon steels this manifests itself at lower phosphorus contents than in low carbon steels. In steels operating only at elevated temperatures, a higher phosphorus content is allowed. About 0.1% P is added to nut and bolt steel to improve machinability. Phosphorus increases the corrosion resistance of steel and prevents sticking of thin sheets when rolling sheet iron.
Chromium is one of the most common alloying elements; it is used both independently and in combination with other elements. The chromium content in alloy steel ranges from 0.5 to 30%. Chromium is a ferrite-forming element; its addition leads to an expansion of the temperature range of metal solidification. At a content of 1.5%, chromium increases the hardness and strength of steel without reducing its ductility. To improve the mechanical properties of steel, about 1% Cr is introduced. Chromium increases the strength of steel at high temperatures and increases oxidation resistance. Steel containing about 5% Cr is heat resistant. In acid-resistant steel, the chromium content is 17-20%, in heat-resistant steel - 23-28%. Chromium increases the hardenability of steel and somewhat reduces the tendency to overheat, increases the abrasion resistance of steel; in an amount of 0.15-0.3% prevents sticking of thin sheets of boiling steel when rolling in batches.
Nickel is used for alloying steel in concentrations from 1 to 25%. It increases strength, especially the toughness of steel and oxidation resistance, increases hardenability, and has little effect on the strength of steel at high temperatures. Nickel is an austenite-forming element. 8-12% Ni is added to acid-resistant steel, and 18-20% to scale-resistant steel; serves as a stabilizer of the austenitic state at high and low temperatures. Nickel is used in large quantities to produce alloys (nichromes) intended for the manufacture of heating elements. Nickel is an expensive and scarce metal, so work is constantly underway to create steels and alloys in which nickel would be replaced by other elements.
Molybdenum for alloying steel is introduced in an amount from 0.2 to 5%. Molybdenum up to 0.6% increases the strength and hardness of steel, improves plastic properties. Molybdenum greatly increases the hardenability of steel and has the property of eliminating temper brittleness. Structural steel contains 0.2-0.4% Mo. Molybdenum increases the strength of steel at high temperatures and therefore it is introduced into heat-resistant (0.4-0.6%) and heat-resistant (2-5%) steels. Some heat-resistant alloys contain more than 5% Mo. Molybdenum is a very expensive and scarce metal, so a lot of research is being done to replace molybdenum in steel with other elements.
Tungsten is used in steels operating at high temperatures and high shock loads. 8.5 and 18% W are added to high-speed tool steel, 1-8% to die and tool steel, and 2-3% to heat-resistant steel. Tungsten is a carbide-forming element, so steel containing tungsten has great strength and hardness. The cost of tungsten is very high, so it is used only for some steels.
Vanadium is a carbide-forming element that greatly refines the austenite grain, increases the strength and toughness of the metal. Steel containing vanadium resists impact loads well. Structural steel contains 0.15–0.4% V, and high-speed tool steel contains 1–2% V. Vanadium is a scarce metal. When processing iron ores containing vanadium, it oxidizes and turns into slag, which is specially processed to extract vanadium.
Titanium forms strong carbides and nitrides and greatly refines the austenite grain. 0.4-0.7% Ti is introduced into acid-resistant steel to bind carbon into strong carbides, as a result of which the tendency of this steel to intercrystalline corrosion decreases. Structural steels contain 0.1–0.15% Ti. Titanium is introduced into steel intended for electric welding in order to reduce self-hardening. In ferritic high-chromium steel, titanium refines the grain and prevents the formation of austenite.
Niobium is a highly carbide-forming element. Increases the strength and hardness of low-alloy steel, and also significantly increases the steel's resistance to oxidation at high temperatures. It is attached to stainless steel to eliminate the tendency to intergranular corrosion, and to carbon (0.1%) and manganese structural steel (0.25%) to eliminate temper brittleness.
Copper increases the strength of ferrite. In an amount of up to 0.5% it increases the ductility of steel in a cold state, in an amount of 0.2% it increases the resistance of carbon steel to atmospheric corrosion. 3-4% Cu is introduced into chromium-nickel stainless steel to increase its corrosion resistance, 0.2% Cu is introduced into steel intended for the manufacture of ship hulls, since copper prevents algae and shells from sticking to the underwater part of the vessel; In addition, copper increases the yield strength of these steels. When the copper content is above 0.3%, areas of a eutectic alloy are formed in the steel, rich in copper and having a low melting point. This alloy is deposited along grain boundaries and causes red brittleness in the metal during forging and rolling.
Boron in an amount of 0.002-0.004% is introduced into structural steel intended for heat treatment in order to increase hardenability. The effect of 0.002% B on increasing hardenability is equivalent to the effect of 0.2% Mo or 1% Ni, therefore boron is introduced instead of scarce elements into high-strength structural steels. The number of steel grades containing boron is increasing every year.
Cobalt is an expensive metal. High-speed steel containing cobalt remains very hard at high temperatures (the cutting edge retains its properties even at red-hot temperatures). Hard magnetic alloys (alnico) contain up to 24% Co. Cobalt increases the resistance of steel against oxidation at high temperatures, and therefore is included in steels from which turbine blades, exhaust valves of internal combustion engines, etc. are made. The cobalt content in the manufactured alloys reaches up to 55%.
Zirconium is introduced into carbon and structural steels. amount 0.1-0.25%. Zirconium, like aluminum, refines the grain of steel, increases the temperature threshold for the onset of grain growth and the hardenability of steel. Increases the endurance limit of steel in air and in a corrosive environment and increases strength characteristics, impact strength at temperatures below zero and improves the weldability of steel. Zirconium increases the heat resistance of steel at temperatures up to 500° C. Complex alloying with zirconium and other elements (vanadium, titanium) affects the properties of steel more strongly than alloying with zirconium alone. Due to its high cost and high waste (~50%), zirconium is not widely used in metallurgy, although several grades of steel have now been developed and recommended to the industry.
Calcium in an amount of 0.2-0.5% is introduced into carbon and structural steels for deoxidation. In high-alloy steels, calcium acts as a modifier. It is usually introduced into steel in the form of silicocalcium, less often in the form of metallic calcium. In the presence of aluminum or rare earth metals, calcium promotes the formation of globular nonmetallic inclusions.
Lead (up to 0.25%) is added to some steels to facilitate cutting. The mechanical properties of steel are affected very little. Lead does not dissolve at all in liquid steel and forms an emulsion; Some of it evaporates when interacting with steel. Lead is placed in molds. Lead oxide fumes are poisonous, so measures must be taken to capture them.
Zinc is used as a coating on thin sheet steel and pipes to protect against rust. It cannot be introduced into liquid steel, since it evaporates at steelmaking temperatures.
Tin is not used as an alloying element, but is used as a coating for very thin (white) tin. Tin enters the steel from the charge. Tin in an amount of 0.06% causes brittleness of steel at forging and rolling temperatures (red brittleness); in amounts up to 0.1% it does not affect the mechanical properties of steel, however, in steel intended for deep drawing, the tin content should not exceed 0.02 -0.03%.
Arsenic gets into steel from iron ores. Especially a lot of arsenic is contained in steel smelted from cast iron obtained from the ores of the Kerch deposit. Arsenic in steel is not a harmful impurity, and its effect is similar to that of copper. At a content of up to 0.1%, arsenic increases the tensile strength and elastic limit of steel (for every 0.01% increase in arsenic content, 0.4 kg/mm2). At the same time, ductility and impact strength are reduced slightly. Up to 0.25% arsenic does not change the weldability of steel. Arsenic, when solidified, is liquidated like sulfur and phosphorus. The addition of arsenic slightly increases the resistance of steel to atmospheric corrosion.
Rare earth metals (cerium, lanthanum, etc.), introduced into steel in the form of mischmetal or ferrocerium, significantly affect the mechanical and technological properties of steels. Cerium and lanthanum are used as modifiers for various steels; at the same time they are desulfurizers and degassers of steel. The amount of mischmetal or ferrocerium introduced into the ladle ranges from 1-3 kg per ton of liquid steel. With the introduction of such a quantity of rare earth metals in ingots and castings of carbon, structural and high-alloy steels, the dendritic structure disappears. At the same time, the fluidity of steel increases, which facilitates the rapid removal of cerium and lanthanum sulfides formed during interaction with liquid metal. The most complete desulfurization of acid steel (approximately 50%) is achieved by introducing cerium (0.2-0.3%) and silicocalcium (0.2-0.3%) directly into the metal stream during release into the ladle. The presence of rare earth metals in steel improves its weldability and deformability in a hot state. The addition of 0.1-0.2% ferrocerium to steel X23N18 helps to refine the cast metal structure and improve the malleability and rollability of ingots. The introduction of 0.05–0.1% misch metal into 40N steel weakens the off-axis zonal heterogeneity of ingots and castings; the addition of 0.15–0.2% alloy practically prevents the formation of whiskers in the ingots. Cerium helps improve the properties of cast steel to the level of forged steel. The more contaminants in liquid steel, the more effective the effect of treating it with cerium. The more alloyed the steel, the lower the optimal amount of additives: cerium. For critical castings made of carbon steel, this value is 0.2-0.3%, for steel alloyed with nickel, chromium, silicon - 0.1-0.15%.
Other elements of the periodic table in the form of impurities may also be present in steel, but their content is so insignificant that they do not have any noticeable effect on the properties of the metal. It is interesting to note that even technically pure iron contains about twenty different elements, although their total content does not exceed 0.25%.