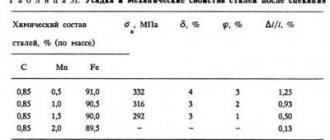
The influence of the main alloying elements on the properties of steel.
The influence of individual components on the properties of steel
Alloy steel is a steel that, in addition to the usual impurities, contains alloying elements specially introduced in certain combinations (Cr, Ni, Mo, Wo, V, A1, B, Ti, etc.), as well as Mn and Si in quantities exceeding their usual content as process impurities (1% and above). As a rule, complex alloying provides the best properties.
Alloying of steels and alloys is used to improve their technological properties. Alloying can increase the yield strength, impact strength, relative contraction and hardenability, and also significantly reduce the hardening rate, the cold brittleness threshold, the deformability of products and the possibility of crack formation. In products with large sections (diameter over 15–20 mm), the mechanical properties of alloy steels are significantly higher than the mechanical properties of carbon steels.
Impact of impurities
Permanent (technological) impurities are essential components of steels and alloys, which is explained by the difficulty of removing them as during smelting (P, S). So in the process of deoxidation (Si, Mn) or from a charge - alloyed scrap metal (Ni, Cr, etc.).
Permanent impurities include carbon, manganese, silicon, sulfur, phosphorus, as well as oxygen, hydrogen and nitrogen.
Carbon
With an increase in carbon content to 1.2%, strength, hardness, cold-brittleness threshold increase (0.1% C increases the temperature of the cold-brittleness threshold by 20C), yield strength, electrical resistance and coercive force. At the same time, density, thermal conductivity, viscosity, plasticity, values of relative elongation and contraction, as well as the value of residual induction are reduced.
A significant role is played by the fact that a change in physical properties leads to a deterioration in a number of technological characteristics - such as deformability during stamping, weldability, etc. Thus, low-carbon steels are characterized by good weldability. Welding medium and especially high-carbon steels requires the use of heating, which slows down cooling, and other technological operations that prevent the formation of cracks.
Manganese
Manganese is introduced into steel as a technological additive to increase the degree of their deoxidation and eliminate the harmful effects of sulfur. Manganese is considered a technological impurity if its content does not exceed 0.8%. Manganese as a technological impurity does not have a significant effect on the properties of steel.
Silicon
Silicon is also introduced into steel for deoxidation. The silicon content as a technological impurity usually does not exceed 0.37%. Silicon as a technological impurity does not affect the properties of steel. In steels intended for welded structures, the silicon content should not exceed 0.12-0.25%.
Sulfur
The limits for sulfur content as a technological impurity are 0.035-0.06%. An increase in sulfur content significantly reduces the mechanical and physicochemical properties of steels, in particular, ductility, impact strength, abrasion resistance and corrosion resistance. During hot deformation of steels and alloys, a high sulfur content leads to red brittleness. In addition, increased sulfur content reduces the weldability of finished products.
Phosphorus
The limits for phosphorus content as a technological impurity are 0.025-0.045%. Phosphorus, like sulfur, is one of the most harmful impurities in steels and alloys. An increase in its content, even by a fraction of a percent, while increasing strength, simultaneously increases fluidity, fragility and the threshold of cold brittleness and reduces ductility and viscosity. The harmful effects of phosphorus are especially pronounced at high carbon levels.
Oxygen and nitrogen
Oxygen and nitrogen dissolve in negligible quantities and contaminate steel with non-metallic inclusions (oxides, nitrides, gas phase). They have a negative impact on properties, causing an increase in brittleness and cold brittleness threshold, and also reduce viscosity and endurance. When the oxygen content is more than 0.03%, steel aging occurs, and more than 0.1% - red brittleness. Nitrogen increases the strength and hardness of steel, but reduces ductility. An increased amount of nitrogen causes strain aging. Aging develops slowly at room temperature and accelerates when heated to 250oC.
Hydrogen
An increase in its content in steels and alloys leads to an increase in brittleness. In addition, flakes may appear in rolled products, which are developed by hydrogen released into the pores. Flocks initiate the destruction process. Metal with flakes cannot be used in industry.
Influence of alloying elements
Alloying of steels and alloys is used to improve their technological properties. Alloying can increase the yield strength, impact strength, relative contraction and hardenability, and also significantly reduce the hardening rate, the cold brittleness threshold, the deformability of products and the possibility of crack formation. In products with large sections (diameter over 15-20 mm), the mechanical properties of alloy steels are significantly higher than the mechanical properties of carbon steels.
All alloying elements, with the exception of nickel, when contained in a solution above a certain limit, reduce impact strength, crack resistance and increase the cold brittleness threshold.
Classification
Based on their applicability for alloying, three groups of elements can be distinguished. The applicability of various elements for alloying is determined not so much by physical, but mainly by economic considerations.
Alloying elements according to the mechanism of their effect on the properties of steels and alloys can be divided into three groups:
- influence on polymorphic (alpha-Fe -> gamma-Fe) transformations;
- formation of carbides (Cr,Fe)7C3 with carbon; (Cr,Fe)23C6; Mo2C and others;
- formation of intermetallic compounds (intermetallic compounds) with iron – Fe7Mo6; Fe3Nb, etc.
Based on the nature of their influence on polymorphic transformations, alloying elements can be divided into two groups:
- elements (Cr, W, Mo, V, Si, Al, etc.), a sufficient content of which ensures the existence of alloyed ferrite (ferrite compounds) in steels at all temperatures;
- elements (Ni, Mn, etc.) that stabilize alloyed austenite at a sufficient concentration at all temperatures (austenitic alloys). Alloys that only partially undergo the gamma->alpha transformation are called semi-austenitic or semi-ferritic, respectively.
Alloying ferrite is accompanied by its hardening. Manganese and chromium have the most significant effect on its strength. Moreover, the finer the ferrite grain, the higher its strength. Many alloying elements help to refine the ferrite and pearlite grains in steel, which significantly increases the toughness of the steel. However, all alloying elements, with the exception of nickel, when contained in a solution above a certain limit, reduce impact strength, crack resistance and increase the cold brittleness threshold. Nickel lowers the threshold of cold brittleness. Alloyed austenite is paramagnetic and has a high coefficient of thermal expansion. Alloying elements, including nitrogen and carbon, the solubility of which in austenite at normal temperatures reaches 1%, increase its strength at normal and high temperatures and reduce the yield strength. Alloyed austenite is the main constituent of many corrosion-resistant, heat-resistant and non-magnetic alloys. It is easily hardened, that is, it is quickly and strongly strengthened under the influence of cold deformation. Alloying elements (with the exception of cobalt), increasing the stability of austenite, reduce the critical hardening rate and increase hardenability. For many austenitic alloys, the critical hardening rate is reduced to 20°C/s and below, which is of great practical importance. Carbide-forming elements: Fe – Mn – Cr – Mo – W – Nb – V – Zr – Ti (with the exception of manganese) prevent the growth of austenite grains when heated. Steel alloyed with these elements, at the same temperature, retains a higher dispersion of carbide particles and, accordingly, greater strength. Intermetallic compounds are formed when there is a high content of alloying elements between these elements or with iron. Examples of such compounds are Fe7Mo6, Fe3Nb2, etc. Intermetallic compounds, as a rule, are distinguished by increased hardness and brittleness.
Optical emission spectral analysis of C, S, P.
Optical emission spectrometers are universal instruments that can solve a wide range of analytical problems. Their work is based on the principles of atomic emission spectral analysis of the elemental composition of matter:
- the spectrum of excited atoms and ions is individual for each element;
- the intensity of the spectral line depends on the concentration of the element in the test sample.
Emission spectral devices are widely used in metallurgy, due to the following advantages of the method:
- Possibility of studying samples in different states of aggregation.
- The analysis is non-destructive.
- The number of elements studied is practically unlimited. These include carbon, sulfur and phosphorus, which are of particular interest to metallurgists.
- To conduct a test, a small amount of a substance is sufficient as a sample.
- High sensitivity and accuracy.
- Expressiveness.
- Possibility of conducting certification analysis.
To analyze carbon, sulfur and phosphorus using emission spectrometers, certain conditions must be created in the device, namely: an oxygen-free atmosphere. Otherwise, it is not possible to determine elements whose wavelength is shorter than 185 nm. Currently, oxygen removal in the device is carried out in two ways:
- by pumping with inert gas;
- vacuuming.
Each deoxygenation system has certain operating and maintenance features, so when choosing a device for analyzing carbon, sulfur and phosphorus, their advantages and disadvantages should be taken into account. This will allow you to select a spectrometer that optimally matches the analytical task, the requirements for the accuracy of research results, and has satisfactory economic indicators.
Optical emission devices involving pumping with inert gas
Argon is most often used in spectral instruments for deoxygenation. To remove oxygen, one of the following systems is provided:
- Open. As a result of purging, oxygen is displaced, and inert gas is removed from the device into the surrounding atmosphere.
- Closed. When the inert gas passes through, oxygen is captured, which is subsequently purified using a filter. The gas continues to move through a closed system, the pressure in which is provided by the pump.
Devices with an open deoxygenation system are characterized by simple design and lower cost. However, in this case, the degree of purification is at a low level, and argon is consumed irrevocably. The use of such spectrometers is advisable when requirements for analytical characteristics are reduced, both on the part of the consumer and on the part of the manufacturer.
The design of devices with a closed deacidification system becomes more complicated, since additional components and their maintenance are required to ensure functionality:
- Pump with power supply.
- Gas cylinder to compensate for losses.
- Additional filter element.
Each of these instrument components requires maintenance and consumables require replacement, which is associated with additional costs. In addition, as a result of unprofessional actions of maintenance personnel, there is a risk of air in the system when replacing the filter. Eliminating the consequences of this requires not only additional material costs, but also time.
Optical emission devices with a vacuum system
The vacuum system allows to obtain a low residual oxygen concentration, which is many times lower than in an open deoxygenation system and comparable to the best results obtained in closed ones. It should be noted that there is no need to use inert gas.
This oxygen removal system is used in the most advanced spectral instruments. They are equipped with an oil pump, which is complemented by special oil traps. In addition, a valve is provided that, in the event of an emergency power outage, prevents damage to the spectrometer by oil as a result of its penetration into the vacuum line.
Two-stage oil foreline pumps are the most preferred equipment compared to oil-free diaphragm models. They have a comparable cost, but at the same time are tens of times superior to the latter in terms of oxygen removal, and also have a significant service life and are much easier to maintain.
Universal desktop and stationary spectrometers Iskroline 100/300 are excellent examples of devices in which a vacuum system is implemented to remove oxygen. They are capable of determining more than 70 elements, which include carbon, sulfur and phosphorus, with a detection limit of up to 0.0001%. The devices allow for quick and accurate spectral analysis of steels, and are characterized by high spectral resolution, high accuracy of measurement results and high quality manufacturing.
The influence of chem. elements on the properties of steel.
Symbols of chemical elements:
| chromium (Cr) - X nickel (Ni) - H molybdenum (Mo) - M titanium (Ti) - T copper (Cu) - D vanadium (V) - F tungsten (W) - B | nitrogen (N) - A aluminum (Al) - Yu beryllium (Be) - L boron (B) - P bismuth (Bi) - Vi gallium (Ga) - Gl | iridium (Ir) - And cadmium (Cd) - Kd cobalt (Co) - K silicon (Si) - C magnesium (Mg) - Ш manganese (Mn) - G | lead (Pb) - AC niobium (Nb) - B selenium (Se) - E carbon (C) - U phosphorus (P) - P zirconium (Zr) - C |
INFLUENCE OF IMPURITIES ON STEEL AND ITS PROPERTIES
Carbon - found in steel usually in the form of a chemical compound Fe3C called cementite. With an increase in carbon content to 1.2%, the hardness, strength and elasticity of steel increase, but ductility and impact resistance decrease, and workability deteriorates, and weldability also deteriorates.
Silicon - if it is contained in steel in small quantities, it does not have a special effect on its properties. (Useful impurity; introduced as an active deoxidizer and remains in the steel in an amount of 0.4%)
Manganese , like silicon, is found in ordinary carbon steel in small quantities and does not have any particular effect on its properties. (A useful impurity; introduced into steel for deoxidation and remains in it in an amount of 0.3-0.8%. Manganese reduces the harmful effects of oxygen and sulfur.
Sulfur is a harmful impurity. It is found in steel mainly in the form of FeS. This compound makes the steel brittle at high temperatures, such as during forging, a property called red brittleness. Sulfur increases the abrasion of steel, reduces fatigue resistance and reduces corrosion resistance. In carbon steel, no more than 0.06-0.07% sulfur is allowed. (Steel is protected from red brittleness by manganese, which binds sulfur into MnS sulfides).
Phosphorus is also a harmful impurity. Reduces viscosity at low temperatures, that is, it causes cold brittleness. Phosphorus somewhat improves the machinability of steel, as it promotes chip separation.
ALLOYING ELEMENTS AND THEIR INFLUENCE ON STEEL PROPERTIES
Chromium (X) is the cheapest and most common element. It increases hardness and strength, slightly reducing ductility, and increases corrosion resistance; the content of large amounts of chromium makes the steel stainless and ensures resistance to magnetic forces
.
Nickel (N) - imparts corrosion resistance
, high strength and ductility,
increases hardenability
, affects the change in the coefficient of thermal expansion. Nickel is an expensive metal; they are trying to replace it with a cheaper one.
Tungsten (B) - forms very hard chemical compounds in steel - carbides, which sharply increase hardness and red-hardness. Tungsten prevents grain growth when heated and helps eliminate brittleness during tempering. This is an expensive and scarce metal.
Vanadium (F) - increases hardness and strength, grinds grain. It increases the density of steel, as it is a good deoxidizer; it is expensive and scarce.
Silicon (C) - in quantities above 1% has a special effect on the properties of steel: a content of 1-1.5% Si increases strength, while the toughness is maintained. With a higher silicon content, electrical resistance and magnetic permeability increase. Silicon also increases elasticity, acid resistance, and scale resistance.
Manganese (G) - with a content above 1% increases hardness
, wear resistance, resistance to shock loads, without reducing ductility.
Cobalt (K) - increases heat resistance, magnetic properties, increases impact resistance.
Molybdenum (M) - increases red hardness, elasticity, tensile strength, anti-corrosion properties and resistance to oxidation at high temperatures
.
Titanium (T) - increases the strength and density of steel
, promotes grain refinement, is a good deoxidizer, improves workability and corrosion resistance.
Niobium (B) - improves acid resistance and helps reduce corrosion in welded structures.
Aluminum (U) - increases heat resistance and scale resistance.
Copper (D) - increases anti-corrosion properties; it is introduced mainly into construction steel.
Cerium - increases strength and especially ductility.
Zirconium (Z) - has a special effect on the size and growth of grain in steel, refines the grain and makes it possible to obtain steel with a predetermined grain size.
Impurities: permanent, hidden and random
Manganese, silicon, aluminum, sulfur and phosphorus
classified as
permanent impurities
.
Aluminum, together with manganese and silicon, is used as a deoxidizer and therefore they are always present in small quantities in deoxidized steels. Iron ores, as well as fuels and fluxes, always contain a certain amount of phosphorus and sulfur, which remain in cast iron and then pass into steel
.
Nitrogen
called
a hidden
impurity - it enters steel mainly from the air.
To random
impurities include
copper, arsenic, tin, zinc, antimony, lead
and other elements. They end up in the steel with the charge - with ores from various deposits, as well as from iron scrap.
All impurities - permanent, hidden and accidental - are inevitable to varying degrees due to steel production technology. Thus, mild steel usually contains these impurities within the following limits: 0.3-0.7% manganese; 0.2-0.4% silicon; 0.01-0.02% aluminum; 0.01-0.05% phosphorus, 0.01-0.04% sulfur, 0.-0.2% copper. In these quantities, these elements are considered as impurities, and in larger quantities, which are intentionally added to steel, they are already considered alloying elements.
Improving steel quality
To remove gases and non-metallic inclusions dissolved in it from liquid steel, vacuum treatment is used. To do this, a ladle with liquid steel is placed in a hermetically sealed chamber, where a vacuum of 267...667 Pa (2...5 mm Hg) is created. The rapidly released gases carry with them non-metallic inclusions and remove them from the metal. Within 10...15 minutes, the amount of dissolved gases decreases by 3...5 times, the number of non-metallic inclusions - by 2...3 times.
To protect the metal from oxidation, steel is cast in an inert atmosphere, for example, argon, under a layer of synthetic slag. To produce steels of particularly high quality, electroslag remelting (ESR), plasma arc remelting, electron beam remelting, and electric arc vacuum remelting are used. The metal is well cleaned (refined) from gases and non-metallic inclusions by treatment with slag and directed crystallization of the liquid melt, creating a deep vacuum.
Dear students! Our website specialists are ready to provide assistance in studying in various subjects: ✔ Solving problems ✔ Completing educational work ✔ Help with exams