Brass is an alloy with copper. The main alloying additive that determines the technological characteristics: strength, flexibility, ductility, good resistance to corrosion processes, etc., is zinc. Additionally, other alloying elements can be introduced, including tin, with the condition that there will be less tin than zinc. In addition to tin, brass may contain nickel, lead, manganese, iron and some other elements in percentages regulated by GOST 15527-2004.
According to the content of chemical elements, brasses are divided into simple (consisting of Cu and Zn) and special (including Cu + Zn, as well as several alloying elements: Pb, Fe, Al, Sn, etc.), according to the type of processing - into deformable ones to create different such as wires, brass sheets, pipes, etc., as well as foundries for the manufacture of parts by casting.
Types of brass metal products
The main types of brass metal products are as follows:
- brass rods - long parts with a round, square, rectangular cross-section;
- brass plates - flat pieces 2.5 cm thick or more;
- brass wire for electrical engineering and other industries;
- brass pipe for carrying communication lines;
- brass circles for the manufacture of machine tools, instrument making, etc.;
- brass sheets for various industries, etc.
For each type of brass metal, a certain grade of metal with a strictly regulated chemical composition is required.
Structure and possible processing methods
The mechanical characteristics of brass of the brand in question, like any other material, are determined by the phase state of its internal structure. In the structure of L63 brass there is no second so-called b-phase, which, if present in a copper alloy, makes it harder and more brittle and significantly impairs the ductility of the base metal. It is the single-phase structure of the alloy of this brand that explains the fact that products made from it are perfectly amenable to pressure processing using almost any of the technologies used today (rolling, drawing, drawing, embossing, bending).
Structure of brass: single-phase (a) and two-phase (b)
Casting methods and cutting technologies are also used to manufacture products from L63 brass, which significantly expands the scope of its application. At industrial enterprises engaged in the production of metals, brass grade L63 is produced in the following form:
- rolled sheets, strips and plates;
- rods with different cross-sectional shapes;
- pipe products;
- wire.
Guaranteed mechanical properties of L63 brass sheets in comparison with L59-1 and copper sheets
The entire range of products made from L63 brass is specified by GOST 15527-70 (new edition - GOST 15527-2004). In addition, as GOST 15527-70 prescribes, manufacturers can produce blanks from a modification of this alloy (L63A), which are distinguished by antimagnetic properties. This alloy, having similar characteristics to L63 brass (specific gravity, density, etc.), has better fluidity in the molten state, but products made from it are not very well processed by cutting.
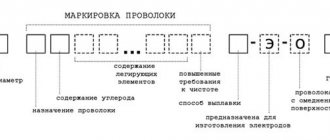
Marking of brass
Based on the content of components, it is customary to distinguish simple and special multi-component brasses. Simple varieties of brass have their own markings, which make it possible to immediately distinguish the name of the alloy brand in the text. The marking includes the letter “L” - brass and a number equal to the average Cu content. L80 brass contains 80% copper and 20% zinc.
For multi-component (special) brasses, the following markings are accepted. The letter “L” also comes first, followed by letters that show all the alloying components of the alloy, except for the main component – zinc. Zinc is not indicated in the labeling name. Following the letters indicating which elements are contained in the alloy, numbers are written that indicate the content of the alloy components. The first number indicates the copper content, then the sequence of numbers corresponds to the sequence of letters in the brand abbreviation. The zinc content is not indicated, it is determined by the difference between 100% and the total content of all other elements.
LAZHMts66-6-3-2 – brass with the following contents:
C – 66%,
Al – 6%,
Fe – 3%,
Mn – 2%.
Zinc content is correspondingly 23%.
In the table below you can see the main brands of brass. They can be cast or deformed for rolled products. It's also worth noting that some types of brass alloys are suitable for soldering. These types are regulated by GOST 16130-90; they are highlighted in color in the table.
| SIMPLE | ALUMINUM | SILICON | TIN | LEAD |
| L96 | LA85-0.5 | LK80-3 | LO90-1 | LS74-3 |
| L90 | LA77-2 | LK62-0.5 | LO70-1 | LS64-2 |
| L85 | LA67-2.5 | LKS65-1.5-3 | LO62-1 | LS63-3 |
| L80 | LAZ60-1-1 | LO60-1 | LS59-1 | |
| L75 | LAN59-3-2 | MANGANESE | LOK59-1-0.3 | LS59-2 |
| L70 | LZhMts59-1-1 | LS58-2 | ||
| L68 | LANKMts75-2-2.5-0.5-0.5 | LMts58-2 | NICKEL | LS58-3 |
| L63 | LMtsA57-3-1 | LN65-5 | LZhS58-1-1 |
Each brand of brass is designed to solve specific problems in accordance with chemical compositions and technological parameters.
Examples of the use of some brands of brass are given below.
Receiving technology
Brass does not occur in nature.
The source material for its production is three types of raw materials:
- Primary. Copper, zinc and other ores are mined from natural deposits.
- Copper, zinc, and other metal scrap suitable for recycling (recyclables). Accumulated at collection points.
- Waste from the metallurgical plants' own production cycle.
Traditional production methods involve the use of furnaces for smelting copper and its alloys. Typically these are electro-induction units equipped with a magnetic core and operating at low frequencies.
Microstructure of ground and etched brass alloy under 400x magnification
Alloy production process:
- Hot copper is placed in a furnace.
- Next, lump zinc is loaded.
- Melting takes place at 875-945°C.
- Alloying additives are added to special brasses.
- The mass is mixed until smooth and poured into molds.
The output is flat or round brass ingots. The smelted products have different hardness, degree of hardening and aging.
Advanced technologies include the installation of ventilation for exhaust during melting of vapors that are dangerous to humans.
Types of brass
It is customary to distinguish single-phase brass or so-called alpha-type brass, containing up to 30-35% zinc, and two-phase varieties of alpha-beta type with a higher (up to 47-50%) content of the main alloying component than in single-phase brasses. Single-phase brasses are more ductile; with increasing additives, the strength of the brass increases, but its ductility significantly decreases.
Two-phase brass alloys are significantly less ductile than single-phase ones. This change in properties due to a change in composition is explained by the fact that with an increase in the number of alloying additives, the structure of the alloy invariably changes. Moreover, the strength of two-phase brass varieties is significantly higher than that of single-phase ones. Dual-phase brass alloys may contain up to 6% lead as an additional alloying additive.
Brass alloys with a relatively low zinc content of up to 10% are usually called tombacs, and with a zinc content of 10-20% - semi-tompacs.
Chemical composition of brass
Brass is close in chemical composition to bronze, and both brass and bronze are based on copper. A significant difference is that the main alloying component in brass alloys is zinc, the content of which can reach 45%.
Let's take a closer look at the properties of the main components of brass.
Zn (zinc) element of the periodic table, atomic number 30. The element belongs to the secondary subgroup 2 of group IV of the IV period. The metal is transitional; it is characterized by the appearance of electrons in d- and f-orbitals in atoms. The metal has a light blue tint, which darkens in air, becoming covered with an oxide film.
Cu is the main component of the alloy. The element belongs to group 11 of the IV period of the Mendeleev periodic system and has atomic number 29. The metal, like zinc, is transitional. The metal has a beautiful yellowish-golden color. When an oxide film forms, copper acquires a reddish tint.
As discussed above, brass can have a structure that consists of an alpha phase or an alpha-beta phase.
As alloying components, brass may include:
- Mn to increase the strength of alloys, including anti-corrosion. In addition to Mn, additional introduction of Al, Sn, and Fe enhances the strength and anti-corrosion characteristics of the metal.
- Sn to improve salt water resistance. Such brass alloys have acquired a “secret” name - marine brass and are widely used in areas of contact with sea water.
- Ni gives the joint high strength characteristics and also increases anti-corrosion properties.
- Pb is used if the brass part will be cut. This element makes the metal more malleable during machining. Brass alloyed with lead is called automatic brass.
- Si is necessary to enhance the antifriction characteristics of the alloy, which makes it possible to safely use it along with bronze in some technological units, bearings, etc. But it is worth noting that silicon significantly reduces the hardness and strength of brass products.
The table below shows the chemical compositions of some brands of brass alloys. The table shows that all brands have different compositions; the copper content in some brands can reach 91%.
Properties of brass depending on the percentage of components, heating temperature
When the percentage of solid solution components changes or additional alloying elements are introduced, the properties of the resulting metal also change.
Let's try to trace how the properties of the metal change when the Zn content changes:
- When the zinc content is less than or equal to 30%, the hardness and elasticity of the metal increase.
- With a further increase in zinc content, the elasticity begins to decrease due to the compaction of the alpha solution. At the same time, hardness increases.
- But when the zinc content reaches 45%, the hardness also drops.
Due to its elasticity, brass can be easily processed under pressure. This especially applies to single-phase alloys. The temperature regime for changing shape should not fall within the range of 300-700°C; this is the “fragile zone” of the metal. Alpha-beta varieties exhibit increased ductility when heating temperature increases above 700°C.
Thus, the content of chemical elements in a metal directly affects its technological parameters and properties. Alpha-brass alloys are characterized by increased ductility, alpha-beta varieties are durable and strong, but they are not suitable for deformation processing. The brass alloy has increased resistance to corrosion and sea water due to the addition of alloying components, which allows its use in areas of constant exposure to aggressive environments.
Decoding the alloy grade and its characteristics
The interpretation of L63 is as follows: L indicates brass, and the numbers indicate the presence of 62-65% copper in the composition. Single-phase structure promotes ease of processing. The production of brass L63 is regulated by GOST 15527-2004; it is used not only for practical but also for decorative purposes.
Among the key characteristics of L63 brass alloys is increased corrosion resistance compared to copper. The material exhibits these qualities:
- in the marine environment;
- in alcohol solutions;
- in fresh water;
- among halogen gases.
The material has good casting characteristics. Suitable for gas and electric welding. Melts at a temperature of 906 °C. During the melting process, components harmful to the human body are formed, so the procedure is carried out in ventilated rooms. The zinc present in the composition is flammable. The wear resistance is increased by hot firing.
This is interesting: Bronze
Features of heat treatment and corrosion resistance
The product in question melts at a temperature of 906oC. In the range from 750oC to 880oC it still exhibits good ductility and can therefore be machined. An important stage in the production of alloy L63 is annealing, which is performed in the range of 550-650oC. As a result of this processing, two main processes occur:
- mechanical stress is relieved;
- metastable phases dissolve to form a single-phase structure.
The presence of mechanical stress is extremely undesirable for L63. It is known that the addition of zinc to copper leads to a significant improvement in its corrosion resistance, therefore all brasses are fairly chemically passive alloys. They are destroyed over time only in aggressive environments, for example in perchloric and nitric acids. However, the presence of stress in brass structures significantly reduces their corrosion resistance.
Due to the formation of the above-mentioned stresses, it is not recommended to subject L63 products to rapid cutting.
What negatively affects the anti-corrosion properties of L63
Sharp processing negatively affects the corrosion resistance of products made from L63 alloy. This happens due to the destruction of the crystalline structure of the material. Cutting also causes a lot of internal stress. Corrosion cracking is often observed on objects made of such material. The reasons for their appearance may be:
- high humidity;
- presence of ammonia in the operating environment;
- heat;
- wet vapors;
- high pressure
To protect against cracking, products are annealed at low temperatures. Corrosion resistance is also affected by contact with mineral acids, mine waters, chlorides, and hydrogen sulfide. Most often, cracking is observed on materials made of thin sheets. For example, on thin-walled pipes and containers. But under conditions of proper use, such products can last a good period.
This is interesting: Soldering copper pipes with your own hands - instructions and video