What characteristics does metal have and how is it processed?
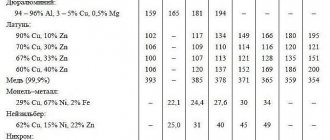
Brass
Brass is an alloy made from copper. In addition to copper, it contains several other components. It is stronger than the parent metal, but has the same good electrical conductivity. The metal is more resistant to oxidation processes and has a decoratively attractive yellow-golden hue.
Depending on the fraction of one or another substance present in the alloy, brass acquires certain properties. The most common brands have a multicomponent structure or consist of two components. In two-component brass, the second metal after copper is zinc, which can be up to 45%. The multicomponent alloy also contains nickel, iron, manganese, and lead.
Chemical composition and properties of brasses
The chemical composition determines the melting point of brass. Zinc plays the main role here. The smaller it is, the higher the temperature at which the substance transitions to the liquid state. For zinc-rich compounds this is +880 degrees, for poor ones - approximately +950 degrees. In the molten state, metals become fluid. Brass flows well when it contains less bismuth and lead. Brands with a lot of these components when melted have increased friability.
Brass is amenable to all basic types of processing. Like bronze, brass is a versatile metal. The first and main thing that can be done with it is to melt it and give it a certain shape using casting. Conveniently, the melting temperature can be easily achieved at home. In addition, brass parts can be welded; they are connected by soldering. Brass alloy also responds well to the mechanical method of processing material using turning equipment.
Properties of brass L63
Properties such as: yield strength, hardness, tensile strength, ductility of L63 brass are higher than those of copper. The alloy has good casting properties. It can be used to make castings. L63, like copper, does not have anti-friction properties. Hot annealing increases the wear resistance of the material. The alloy is perfectly amenable to pressure processing: deep drawing, rolling, embossing, bending without significant consequences, drawing.
Alloy L63 is processed worse by cutting than LS59-1, but better than duralumin, copper and most bronze alloys. It can be turned without problems. The melting point of L63 is 906 °C. To carry out brass smelting, it is necessary to use a special exhaust and ventilation system. During the melting process, harmful substances are released that are dangerous to human health, and the alloy components can ignite in air (for example, zinc). The alloy is amenable to gas and electric welding, and can be soldered with hard and soft solders.
What tools are needed to melt brass
Melting steel in induction furnaces
To melt brass before subsequent casting, it must be heated to almost +900 degrees Celsius. To do this, you will need to purchase or make a special oven. An induction type oven heats up most efficiently.
The frame of a homemade forge can be assembled from refractory bricks with a bond of refractory clay, and be sure to be tied with metal to avoid destruction of the structure at elevated temperatures inside. A removable lid is placed on top.
The interior decoration of the stove is an electric spiral, evenly fixed along the entire perimeter of the walls using ceramic tubes. The device where metal parts are directly placed is called a crucible. This is a special container that can withstand high thermal loads. Therefore, it is not made of metal. Factory models of crucibles are often made of graphite. For home use, you can make a crucible from fireclay bricks.
To create the required heat inside the furnace, the power of the electric coil must be designed for at least 20 kilowatts. When making a spiral yourself, when choosing nichrome wire, you need to take this point into account. Additional tools you need to have to work with molten brass:
- Tongs for removing a hot crucible with metal from the furnace of the furnace;
- Steel spoon to remove film and slag from the melt;
- A casting ladle into which liquid brass is poured before pouring the mold;
- Personal protective equipment for vision, breathing and skin;
- Fire extinguishing agents (fire extinguisher).
In addition to all of the above, the room in which the brass composition is melted must be equipped with supply and exhaust ventilation - zinc vapor is very harmful to the body.
Temperature conditions, melting technology
You need to understand that the melting temperature of brass and the casting temperature are different. In order for the metal to flow well and fill the smallest cavities of the mold, it is not enough to simply melt it. This is especially true for lead brasses, because lead, as mentioned earlier, impairs the fluidity of the substance. Therefore, tables for different brands of brass provide several temperature processing conditions.
Heat treatment of brasses with lead
The technological table for grades of lead-containing brass shows both the melting temperature of the metal and the casting temperature:
- LS59-1V – melting: 900 degrees Celsius, casting: 1030-1080 degrees;
- LS59-1 – melting: 885-895 (casting: 1030-1080);
- LS60-1 – melting: 885-900 (casting: 1040-1080);
- LS63-3 – melting: 885-905 (casting: 1060-1100);
- LS64-2 – melting: 885-910 (casting: 1060-1100);
- LS74-3 – melting: 965 (casting: 1120-1160).
Heat treatment of plain brasses
For simple alloys, only the melting point of brass is indicated:
- L60 – 885-895 degrees Celsius;
- L63 – 900-910;
- L68 – 909-938;
- L70 – 915-950;
- L80 – 965-1000;
- L85 – 990-1025;
- L90 – 1025-1045;
- L96 – 1055-1070.
Plain brass - chemical composition and application
Stages of smelting work
Preparing the workplace is a preliminary and important step for effectively carrying out brass smelting work at home. The surface of the table where the stove will be installed is covered with an asbestos sheet. The latter acts as a thermal barrier. All the necessary tools are located right there. Forms for pouring should be laid out on a separate table.
The next stage of work is the preparation of the metal. It is cleaned of dirt, strong oxides and degreased. All this reduces the amount of slag in the molten mass. Next comes the process of grinding the material. The smaller the fractions, the faster the brass will reach a fluid state. The resulting particles are poured into a crucible and lowered into an induction furnace.
Brass melting process
After turning on the heating element of the furnace, a certain time must pass before the pieces of metal turn into a viscous mass, and then into a molten liquid. The substance must not be overheated, otherwise intense evaporation of zinc will occur, which will worsen the chemical properties after hardening. Therefore, the process is constantly monitored, periodically lifting the oven lid. The finished melt should have a bright yellow color with an orange tint.
The oxidizing film is removed from the surface of the melt, the slag is removed, being careful not to mix the mass. Using tongs, carefully take the crucible and pour the liquid alloy into pre-prepared molds. To avoid the formation of shells in a frozen part, which occur when the melting temperature of brass sharply drops, and the inability of air bubbles to escape, it is advisable to warm up the molds before pouring using a technical hair dryer.
Safety requirements
- It is advisable to melt brass outdoors under a canopy. If the workplace is indoors, provide good ventilation;
- There should be no flammable objects, explosive liquids, or gas cylinders in the immediate vicinity of the stove;
- You must have a fire extinguisher and a large container of water in an accessible place near your desk;
- The work is carried out in overalls made of cotton fabric, shoes should be made of thick leather;
- The visual organs are protected with glasses, the respiratory organs with a respirator;
- On the approach to the workplace, tables with casting molds, there should be no foreign objects or wires that can get caught;
- When pouring molten metal into a mold, you should try to avoid splashes;
- The premises must have a first aid kit containing everything necessary to provide first aid.
Anti-corrosion properties of brass L63
Brass L63 has good anti-corrosion properties in fresh and sea water, dry steam, alcohols, antifreeze and freons, as well as in air. Alloy L63 loses its resistance to corrosion after cutting or machine processing. This is due to disturbances in the crystal structure of the alloy composition and residual stress in the metal. Factors that catalyze the corrosion cracking process are: excess moisture, high temperature, and the presence of sulfur dioxide and ammonia in the atmosphere. To prevent cracking, all products made from L63 are recommended to be annealed at low temperatures.
Brass L63 is sensitive to corrosion cracking. This process can be triggered by factors such as traces of ammonia, the presence of moisture and sulfur dioxide in the air. This is a seasonal phenomenon depending on humidity. Its intensity varies at different times of the year. Annealing at low temperatures helps prevent cracking of L63 brass products.
Limitations on the corrosion resistance of the alloy are observed upon contact with fatty and mineral acids, oxidizing solutions, chlorides and hydrogen sulfide, in mine waters, as well as at high pressure in humid and saturated vapors. Products made from thin sheets are most prone to corrosion cracking and oxidation processes: tanks, tanks, thin-walled pipes. When used correctly, thin-walled brass products are successfully used in various fields of industry.