Copper is a pink-red metal, belongs to the group of heavy metals, and is an excellent conductor of heat and electric current. The electrical conductivity of copper is 1.7 times higher than that of aluminum and 6 times higher than that of iron.
Copper.
It has a characteristic reddish color and is found in nature in the form of sulfur compounds, in oxides and very rarely in pure form. Copper is marked with the letter M. Depending on the purity of the copper (GOST 859-2001). The purest copper contains 99.99% copper and 0.01% impurities. Due to its high ductility, copper is well processed by pressure in cold and hot states. It has good electrical conductivity. Conductors of electric current - wires and cables - are made from it.
Chemical properties of copper
Copper is a low-active metal that does not interact with water, alkali solutions, hydrochloric and dilute sulfuric acid. However, copper dissolves in strong oxidizing agents (for example, nitrogen and concentrated sulfur).
Copper has a fairly high resistance to corrosion. However, in a humid atmosphere containing carbon dioxide, the surface of the metal becomes covered with a greenish coating (patina).
Basic physical properties of copper
Melting point °C
Boiling point °C
Density, γ at 20°C, kg/m³
Specific heat capacity at constant pressure, Ср at 20°C, kJ/(kg•J)
Temperature coefficient of linear expansion, а•10 6 from 20 to 100°C, K -1
Electrical resistivity, р at 20°C, μΩ•m
Thermal conductivity λ
at 20°C, W/(m•K)
Specific electrical conductivity, ω
at 20°C, MOhm/m
Chemical properties
The chemical properties of copper are determined by its position in the periodic table, where it has serial number 29 and is located in the fourth period. What is noteworthy is that it is in the same group with noble metals. This once again confirms the uniqueness of its chemical properties, which should be discussed in more detail.
Shades of copper alloys
In conditions of low humidity, copper exhibits virtually no chemical activity. Everything changes if the product is placed in conditions characterized by high humidity and high carbon dioxide content. Under such conditions, active oxidation of copper begins: a greenish film consisting of CuCO3, Cu(OH)2 and various sulfur compounds is formed on its surface. This film, called patina, performs the important function of protecting the metal from further destruction.
Oxidation begins to actively occur when the product is heated. If the metal is heated to a temperature of 375 degrees, then copper oxide is formed on its surface, if higher (375-1100 degrees) then two-layer scale.
Copper reacts quite easily with elements that are part of the halogen group. If a metal is placed in sulfur vapor, it will ignite. It also shows a high degree of affinity for selenium. Copper does not react with nitrogen, carbon and hydrogen even at high temperatures.
The interaction of copper oxide with various substances deserves attention. Thus, when it reacts with sulfuric acid, sulfate and pure copper are formed, with hydrobromic and hydroiodic acid - copper bromide and iodide.
The reactions of copper oxide with alkalis, which result in the formation of cuprate, look different. The production of copper, in which the metal is reduced to a free state, is carried out using carbon monoxide, ammonia, methane and other materials.
Copper, when interacting with a solution of iron salts, goes into solution, and the iron is reduced. This reaction is used to remove the deposited copper layer from various products.
Mono- and divalent copper is capable of creating complex compounds that are highly stable. Such compounds are double copper salts and ammonia mixtures. Both have found wide application in various industries.
Copper wire coils
Mechanical properties of copper
Tensile strength, σ MPa
Relative elongation after break, δ ψ%
Relative narrowing after rupture, %
Brinell hardness, HB
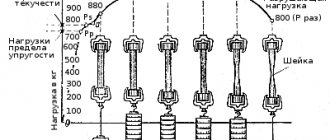
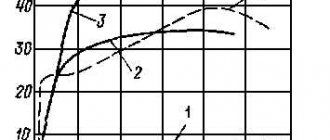
At negative temperatures, copper has higher strength properties and higher ductility than at a temperature of 20°C. Commercial copper has no signs of cold brittleness. As the temperature decreases, the yield strength of copper increases and the resistance to plastic deformation increases sharply.
Interesting things about copper
Initially, the process of recovering this metal looked very primitive: copper ore was simply heated over fires and then subjected to sudden cooling, which led to cracking of pieces of ore, from which copper could already be extracted. Further development of this technology led to the fact that air began to be blown into the fires: this increased the heating temperature of the ore. Then the ore began to be heated in special structures, which became the first prototypes of shaft furnaces.
The fact that copper has been used by mankind since ancient times is evidenced by archaeological finds, as a result of which products made from this metal were found. Historians have established that the first copper products appeared already in the 10th millennium BC, and it began to be most actively mined, processed and used 8–10 thousand years later. Naturally, the prerequisites for such active use of this metal were not only the relative ease of its extraction from ore, but also its unique properties: specific gravity, density, magnetic properties, electrical and specific conductivity, etc.
Nowadays, it is already difficult to find copper in nature in the form of nuggets; it is usually mined from ore, which is divided into the following types.
- Bornite - this ore can contain copper in amounts up to 65%.
- Chalcocite, also called copper luster. Such ore can contain up to 80% copper.
- Copper pyrite, also called chalcopyrite (content up to 30%).
- Covelline (content up to 64%).
Chalcopyrite
Copper can also be extracted from many other minerals (malachite, cuprite, etc.). They contain it in different quantities.
Copper Applications
Due to their valuable qualities, copper and copper alloys are used in the electrical and electrical engineering industries, in radio electronics and instrument making. There are alloys of copper with metals such as zinc, tin, aluminum, nickel, titanium, silver, and gold. Less commonly used are alloys with non-metals: phosphorus, sulfur, oxygen. There are two groups of copper alloys: brass (alloys with zinc) and bronze (alloys with other elements).
Copper is highly environmentally friendly, which allows its use in the construction of residential buildings. For example, a copper roof, due to its anti-corrosion properties, can last more than a hundred years without special care or painting.
Copper in alloys with gold is used in jewelry. This alloy increases the strength of the product, increases resistance to deformation and abrasion.
Copper compounds are characterized by high biological activity. In plants, copper takes part in the synthesis of chlorophyll. Therefore, it can be seen in the composition of mineral fertilizers. A lack of copper in the human body can cause deterioration in blood composition. It is found in many food products. For example, this metal is found in milk. However, it is important to remember that excess copper compounds can cause poisoning. This is why you should not cook food in copper cookware. During boiling, large amounts of copper can leach into food. If the dishes inside are covered with a layer of tin, then there is no danger of poisoning.
In medicine, copper is used as an antiseptic and astringent. It is a component of eye drops for conjunctivitis and solutions for burns.
Modulus of Elasticity (Young's Modulus)
If a product made of a certain material is subjected to a certain force, it begins to resist this action: compress, stretch or bend. The ability for such opposition can be assessed and expressed mathematically. The name of this strength characteristic is elastic modulus.
The parameter is different for each material and characterizes its strength. They use the value when developing structures, parts and other products in order to prevent violation of their integrity.
Strength modules
In addition to normal loading, there are other force effects on materials.
The shear modulus G determines the stiffness. This characteristic shows the maximum load value for changing the shape of an object.
The bulk modulus of elasticity K determines the elastic properties of a material to change volume. With any deformation, the shape of the object changes.
For different steels, the values of the indicated modules are given in Table 3.
Table 3: Strength moduli for steels
| Name of steel | Young's modulus of elasticity, 10¹² Pa | Shear modulus G, 10¹² Pa | Modulus of bulk elasticity, 10¹² Pa | Poisson's ratio, 10¹²·Pa |
| Low carbon steel | 165…180 | 87…91 | 45…49 | 154…168 |
| Steel 3 | 179…189 | 93…102 | 49…52 | 164…172 |
| Steel 30 | 194…205 | 105…108 | 72…77 | 182…184 |
| Steel 45 | 211…223 | 115…130 | 76…81 | 192…197 |
| Steel 40Х | 240…260 | 118…125 | 84…87 | 210…218 |
| 65G | 235…275 | 112…124 | 81…85 | 208…214 |
| X12MF | 310…320 | 143…150 | 94…98 | 285…290 |
| 9ХС, ХВГ | 275…302 | 135…145 | 87…92 | 264…270 |
| 4Х5МФС | 305…315 | 147…160 | 96…100 | 291…295 |
| 3Х3М3Ф | 285…310 | 135…150 | 92…97 | 268…273 |
| R6M5 | 305…320 | 147…151 | 98…102 | 294…300 |
| P9 | 320…330 | 155…162 | 104…110 | 301…312 |
| P18 | 325…340 | 140…149 | 105…108 | 308…318 |
| R12MF5 | 297…310 | 147…152 | 98…102 | 276…280 |
| U7, U8 | 302…315 | 154…160 | 100…106 | 286…294 |
| U9, U10 | 320…330 | 160…165 | 104…112 | 305…311 |
| U11 | 325…340 | 162…170 | 98…104 | 306…314 |
| U12, U13 | 310…315 | 155…160 | 99…106 | 298…304 |
For other materials, strength characteristics are indicated in specialized literature. However, in some cases individual studies are carried out. Such research is especially relevant for building materials. At enterprises where reinforced concrete products are produced, tests are regularly carried out to determine limit values.
Young's modulus
Young's modulus (modulus of elasticity) is a physical quantity that characterizes the properties of a material to bend or stretch under the influence of force;
in fact, the rigidity of the body depends on this. This is a property of any material, and it depends on the temperature and pressure applied.
In physics, elasticity is the property of solid materials to return to their original shape and size after the forces applied during deformation are removed.
In other words: when a body is deformed, a force appears that strives to restore the original shape and size of the body. The elastic force is this manifested force. It is also a consequence of electromagnetic interaction between particles.
A low value of Young's modulus means that the solid being studied is elastic .
A high value of Young's modulus means that the solid being studied is inelastic or rigid.
Examples of Young's modulus (elasticity) values for:
- (i.e. for rubber it is 5 times less than steel)
Copper production
Copper is mined from oxide and sulfide ores. 80% of all mined copper is smelted from sulfide ores. Typically, copper ores contain a lot of gangue. Therefore, a beneficiation process is used to obtain copper. Copper is obtained by smelting it from sulfide ores. The process consists of a number of operations: roasting, smelting, converting, fire and electrolytic refining. During the firing process, most of the impurity sulfides are converted into oxides. Thus, the main impurity of most copper ores, pyrite FeS2, turns into Fe2O3. The gases produced during roasting contain CO2, which is used to produce sulfuric acid. The resulting oxides of iron, zinc and other impurities during the firing process are separated in the form of slag during melting. Liquid copper matte (Cu2S with an admixture of FeS) enters the converter, where air is blown through it. During conversion, sulfur dioxide is released and crude or raw copper is obtained. To extract valuable (Au, Ag, Te, etc.) and to remove harmful impurities, blister copper is first subjected to fire and then electrolytic refining. During fire refining, liquid copper is saturated with oxygen. In this case, impurities of iron, zinc and cobalt are oxidized, turn into slag and are removed. And copper is poured into molds. The resulting castings serve as anodes during electrolytic refining.
The main component of the solution during electrolytic refining is copper sulfate - the most common and cheapest copper salt. To increase the low electrical conductivity of copper sulfate, sulfuric acid is added to the electrolyte. And to obtain a compact copper deposit, a small amount of additives is introduced into the solution. Metal impurities contained in crude (“blister”) copper can be divided into two groups.
- Fe, Zn, Ni, Co. These metals have significantly more negative electrode potentials than copper. Therefore, they anodicly dissolve together with copper, but are not deposited on the cathode, but accumulate in the electrolyte in the form of sulfates. Therefore, the electrolyte must be replaced periodically.
- Au, Ag, Pb, Sn. Noble metals (Au, Ag) do not undergo anodic dissolution, but during the process they settle at the anode, forming anode sludge together with other impurities, which is periodically removed. Tin and lead dissolve together with copper, but in the electrolyte they form poorly soluble compounds that precipitate and are also removed.
Modulus of elasticity of various materials
Elastic moduli for different materials have completely different values, which depend on:
- the nature of the substances that form the composition of the material;
- mono- or multicomponent composition (pure substance, alloy, etc.);
- structures (metallic or other type of crystal lattice, molecular structure, etc.);
- density of the material (distribution of particles in its volume);
- the processing to which it was subjected (firing, etching, pressing, etc.).
For example, in the reference data you can find that the elastic modulus for aluminum is in the range from 61.8 to 73.6 GPa. Apparently, this depends on the state of the metal and the type of processing, because for annealed aluminum the Young’s modulus is 68.5 GPa.
Its significance for bronze materials depends not only on the processing, but also on the chemical composition:
- bronze – 10.4 GPa;
- aluminum bronze during casting – 10.3 GPa;
- rolled phosphor bronze – 11.3 GPa.
Young's modulus of brass is much lower - 78.5-98.1. Rolled brass has the maximum value.
Copper itself in its pure form is characterized by a resistance to external influences that is much greater than its alloys - 128.7 GPa. Processing it also reduces the indicator, including rolling:
- cast – 82 GPa;
- rolled – 108 GPa;
- deformed – 112 GPa;
- cold drawn – 127 GPa.
Titanium (108 GPa), which is considered one of the strongest metals, has a value close to copper. But heavy but brittle lead shows only 15.7-16.2 GPa, which is comparable to the strength of wood.
For iron, the stress-to-strain ratio also depends on the method of its processing: cast - 100-130 or forged - 196.2-215.8 GPa.
Cast iron is known for its brittleness and has a stress-to-strain ratio of 73.6 to 150 GPa, which is consistent with its type. Whereas for steel, the elastic modulus can reach 235 GPa.
Elastic moduli of some materials
The values of strength parameters are also influenced by the shape of the products. For example, for a steel rope, calculations are carried out, which take into account:
- its diameter;
- lay pitch;
- lay angle.
Interestingly, this figure for a rope will be significantly lower than for a wire of the same diameter.
It is worth noting the strength and non-metallic materials. For example, among the Young's moduli of wood, pine has the lowest - 8.8 GPa, but the group of hardwoods, which are united under the name "iron wood", has the highest - 32.5 GPa, oak and beech have equal indicators - 16.3 GPa.
Among building materials, the resistance to external forces of seemingly durable granite is only 35-50 GPa, while even glass is 78 GPa. Concrete is inferior to glass - up to 40 GPa, limestone and marble, with values of 35 and 50 GPa, respectively.
Flexible materials such as rubber can withstand axial loads ranging from 0.0015 to 0.0079 GPa.
Round copper rods (according to GOST 1535-91 as amended in 2001)
The standard applies to drawn copper bars of round, square, hexagonal cross-section and pressed round bars.
- Diameters of pressed (hot-rolled) round bars, mm:
20, 22, 28, 30, 32; 35; 38; 40; 45; 50; 55; 60; 65; 70; 75; 80; 85; 90; 95; 100, 110, 120, 130, 140, 150. - Diameters of drawn rods, mm:
3; 3.5; 4; 4.5; 5; 5.5; 6; 7; 8; 9; 10; eleven; 12; 13; 14; 15; 16; 17; 18; 20; 21; 22; 24; 25; 27; 28; thirty; 32; 33; 35; 36; 38; 40; 41; 45; 46; 50. The diameter of the inscribed circle is taken as the diameter of square and hexagonal rods.
Drawn rods are produced
- soft (annealed) - M,
- semi-solid - PT,
- hard - T;
by accuracy:
- high - B,
- increased - P,
- normal - N.
GOST 1535-91 provides dimensions
pressed round and drawn round, square and hexagonal rods.
The rods are made from copper grades
M1, M1r, M2, M2r, M3 and M3r.
M1E grade copper is used only for the manufacture of conductive parts.
Examples of notation:
- Drawn rod (D), round (CR), high-precision manufacturing (B), solid (T), diameter 10 mm, unmeasured length (ND) from copper M1, for processing on automatic machines (AB):
Rod DKRVT 10ND M1 AB
GOST 1535-91
- The same, hexagonal (SH), high precision (P), soft (M), diameter 19 mm, length 3000 mm, made of copper M2:
Rod DShGPM 19 x 3000 M2
GOST 1535-91.
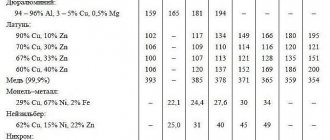
Percentage composition of copper M1
| Element | Content |
| Pb | up to 0.005 |
| Ag | up to 0.003 |
| Zn | up to 0.004 |
| Fe | up to 0.005 |
| S | up to 0.004 |
| As | up to 0.002 |
| Cu | 99,9 |
| Ni | up to 0.002 |
| Fe | up to 0.005 |
| S | up to 0.004 |
| As | up to 0.002 |
| Cu | 99,9 |
General characteristics of copper and its alloys
Copper is a metal without polymorphic transformations with a fcc crystalline lattice. The melting point is 1083°C. When heated, there are no polymorphic transformations in copper. The density of copper is 8940 kg/m3.
Copper is easily polished, soldered and welded well. In terms of electrical conductivity and thermal conductivity, copper ranks second after silver. Copper has high corrosion resistance in atmospheric conditions, fresh and sea water, caustic alkalis, organic acids and other aggressive environments, but interacts with ammonia and sulfur dioxide gases.
Copper is well rolled into thin sheets and strips. Cold plastic deformation (reaching 90% or more) increases the strength, hardness, and elastic limit of copper, but reduces ductility and electrical conductivity. During plastic deformation, a texture appears that causes anisotropy in the mechanical properties of copper. Annealing to remove hardening is carried out at 550 – 600°C in a reducing atmosphere, since copper easily oxidizes when heated.
The disadvantages of copper include: low strength, poor machinability and low fluidity.
Depending on the content of impurities, the following grades of copper are distinguished: M00 (99.99% Cu), M0 (99.97% Cu), M1 (99.9% Cu), M2 (99.7% Cu), M3 (99. 5% Cu).
The most commonly found elements in copper are divided into three groups.
The first group includes copper-soluble elements Al, Fe, Ni, Sn, Zn, which increase the strength and hardness of copper and are used for alloying copper-based alloys. These impurities sharply reduce the electrical and thermal conductivity of copper.
The second group consists of copper-insoluble elements Pb and Bi, which worsen the mechanical properties of copper and single-phase alloys based on it. Forming low-melting eutectics (at 326 and 270°C, respectively), located along the grain boundaries of the main phase, they cause red brittleness of the alloys.
Insoluble elements O, S, Se, Te are present in copper and its alloys in the form of intermediate phases (for example, Cu2O, Cu2S). They constitute the third group of elements and form eutectics with copper with a high melting point that do not cause red brittleness. Oxygen when annealing copper in hydrogen causes “hydrogen disease”, which can lead to destruction of the metal during pressure treatment or operation of finished parts.
To alloy copper alloys, elements soluble in copper are mainly used - Zn, Sn, Al, Be, Si, Mn, Ni. By increasing the strength of copper alloys, alloying elements practically do not reduce, and some of them (Zn, Sn, Al) increase ductility. High ductility is a distinctive feature of copper alloys. The relative elongation of some single-phase alloys reaches 65%. Copper alloys are inferior in strength to steel. The tensile strength of most copper alloys lies in the range of 300...500 MPa, which corresponds to the properties of low-carbon unalloyed steels in a normalized state. And only the most durable beryllium bronzes after hardening and aging have a tensile strength of 1100...1200 MPa and corresponds to the strength level of medium-carbon alloy steels subjected to thermal improvement.
Copper alloys are divided into two main groups: brass and bronze.
Copper alloys are labeled by chemical composition, using letters to designate elements and numbers to indicate their mass parts. In copper alloys, aluminum is designated by the letter A, beryllium - B, iron - F, silicon - K, magnesium - Mg, nickel - N, tin - O, lead - C, phosphorus - F, zinc - C, zirconium - Cr, chromium - X; manganese – Mts.
Brasses (alloys of copper and zinc) are marked with the letter L
.
In deformable brasses, which do not contain other elements besides copper and zinc, the letter L
is followed by a number indicating the average copper content.
In multi-component brasses, after L
there are letters - symbols of the elements, and then numbers indicating the content of copper and each alloying element.
For example, L68
contains 68% Cu,
LAN59-3-2
contains 59% Cu, 3% Al;
2% Ni (rest Zn). In grades of foundry brass, the zinc content is indicated, and the amount of each alloying element is placed directly after the letter indicating it. For example, brass LTs40Mts3A
contains 40% Zn, 3% Mn and 1% Al.
Bronzes (alloys of copper with all elements except zinc) are designated by the letters Br
, followed by letters and numbers.
In stamps of wrought bronzes, letters are first placed - symbols of alloying elements, and then numbers indicating their content. For example, BrAZh9-4
contains 9% A1, 4% Fe, the rest is Cu.
In grades of cast bronzes, after each letter the content of this alloying element is indicated. For example, BrO6Ts6S3
contains 6% Sn, 6% Zn, 3% Pb, the rest is Cu.
Brass
Copper with zinc forms an a-solid solution with a limiting zinc concentration of 39% (Fig. 22). With a higher zinc content, an electronic compound CuZn (β-phase) with a bcc crystal lattice is formed. At 454...468°C (dashed line in the diagram), ordering of the β-phase occurs, and the ordered β-solid solution is designated as the β'-phase.
Figure 22 — Cu-Zn phase diagram
If there is only an α-solid solution in the brass structure, an increase in the zinc content causes an increase in its strength and ductility. The appearance of the β'-phase is accompanied by a sharp decrease in plasticity and an increase in hardness and brittleness. Strength continues to increase as the zinc content increases to 45% while the brass is in the two-phase state. With a further increase in the percentage of zinc, brass transitions to a single-phase state with a β'-phase structure, which causes a sharp decrease in strength. Brasses containing up to 45% Zn are of practical importance. Alloys with a high zinc content are highly brittle.
Double brasses (containing only copper and zinc) are divided into single-phase (with an α-solid solution structure) and two-phase (with an α and β-phase structure).
Single-phase brasses have high ductility and respond well to cold plastic deformation, which significantly increases their strength and hardness. To increase plasticity, recrystallization annealing is carried out at 600...700°C.
Parts that are made of wrought brass with a content of more than 20% zinc may be subject to “seasonal” cracking in humid air in the presence of sulfur gases in the atmosphere. To prevent cracking, parts are annealed at temperatures below the recrystallization temperature (in most cases at 250...270°C).
Increasing the zinc content makes brass cheaper, improves its machinability and ability to resist wear, but at the same time, thermal conductivity and electrical conductivity decrease.
Impurities increase the hardness and reduce the ductility of brass. Lead and bismuth are especially unfavorable, causing red brittleness in single-phase brasses. Therefore, single-phase brasses are not subjected to hot deformation, but are produced in the form of cold-rolled strips, tapes, wires, and sheets. Rolled steel is used to produce parts using the deep drawing method (radiator tubes, shell casings, bellows, pipelines), as well as parts that require low hardness due to operating conditions (washers, bushings, O-rings, etc.).
Due to the short crystallization temperature range, double brasses have a low tendency to dendritic segregation, high fluidity, low diffuse shrinkage porosity and good tightness. But, despite this, they are practically not used for shaped castings, since they have a rather large concentrated shrinkage cavity.
To increase machinability, lead is added to brass. Brass LS59-1 (“automatic”) is supplied in rods and is intended for the manufacture of parts on automatic machines.
Al, Fe, Ni, Sn, Si are used to alloy brass. These elements increase the strength and corrosion resistance of brass. Therefore, alloyed brasses are widely used in river and marine shipbuilding (LAZH60-1-1). Brasses alloyed with tin (LO70-1, LO62-1) have high corrosion resistance in sea water and therefore are called “marine brasses”.
Practical applications are found in brass with the addition of aluminum up to 4% (LA77-2), which, due to their single-phase structure, can be easily processed under pressure.
Aluminum brasses are additionally alloyed with nickel, iron, manganese, silicon, which have variable solubility in α-solid solution, which allows these brasses to be strengthened by hardening and aging.
Brass LANKMts75-2-2.5-0.5-0.5 is the only alloy based on the Cu-Zn system, which is strengthened by dispersion hardening. The hardening temperature is 800°C, after which brass has high ductility, and after aging it acquires high strength (sв = 700 MPa, d = 25%). Good ductility in the hardened state allows the alloys to be further strengthened by plastic deformation before aging, which ensures an increase in tensile strength up to 1000 MPa.
Silicon brasses are characterized by high strength, ductility, and toughness both at normal and low temperatures (up to -183°C). When alloying brasses, small additions of silicon (LK80-3) are used to obtain a single-phase structure. Such brasses are used for the manufacture of fittings, instrument parts, in shipbuilding and mechanical engineering.
Bronze
4.5.3.1 Tin bronzes
The limiting solubility of tin in copper corresponds to 15.8%, but at a tin concentration of more than 10%, a δ-phase is formed in the structure of bronzes, causing a sharp decrease in their viscosity and ductility. Therefore, bronzes containing only up to 10% Sn are of practical importance.
Among copper alloys, tin bronzes have the lowest linear shrinkage, which makes it possible to produce complex shaped castings. Double and low alloy casting bronzes contain 10% Sn. Tin bronzes are alloyed with Zn, Pb, P (BrO3Ts12S5, BrO4Ts4S17, BrO10Ts2, etc.). Zinc completely dissolves in an α-solid solution when alloyed to 15% and, by reducing the crystallization interval of tin bronzes, improves their fluidity and density of castings. Lead increases the anti-friction properties and improves the machinability of tin bronzes. Phosphorus, being a deoxidizer of tin bronzes, increases their fluidity, and wear resistance improves due to the appearance of solid inclusions of copper phosphide Cu3P.
High corrosion resistance in atmospheric conditions, fresh and sea water contributes to the widespread use of casting bronzes for steam-water fittings operating under pressure.
Deformable bronzes contain up to 6...8% Sn (BrOF 4-2.5, BrOTs 4-3, etc.). To eliminate dendritic segregation and level out the chemical composition, as well as improve workability under pressure, diffusion annealing is used, which is carried out at 700...750°C. During cold plastic deformation, bronze is subjected to intermediate annealing at 550...700°C. Wrought bronzes are characterized by good ductility and higher strength than cast bronzes.
Deformable bronzes have high elastic properties and fatigue resistance. They are used for the manufacture of round and flat springs in precision mechanics, electrical engineering, chemical engineering and other areas of industry.
4.5.3.2 Aluminum bronzes
Aluminum bronzes are distinguished by high mechanical, anti-corrosion and anti-friction properties. Their advantages over tin bronzes are lower cost and higher mechanical and technological properties. In particular, a short crystallization interval provides aluminum bronzes with high fluidity, concentrated shrinkage and good tightness of castings, and a low tendency to dendritic segregation.
Copper and aluminum form an α-solid solution. With an increase in aluminum content to 4.5%, along with strength and hardness, ductility increases, which then drops sharply, and strength continues to increase with an increase in aluminum content to 10...11%. With the appearance of a eutectoid containing hard and brittle phases at these concentrations, wear resistance increases and antifriction properties appear.
Single-phase bronzes (BrA5, BrA7) are deformable. They have the best combination of strength (σв = 400...450 MPa) and ductility (δ = 60%).
Two-phase bronzes are distinguished by high strength (σв = 600 MPa) and hardness (> 100 HB). They can be subjected to strengthening heat treatment.
Aluminum bronzes are alloyed with iron, nickel, and manganese. In the α-phase of aluminum bronze, up to 4% iron is dissolved; at higher contents, Al3Fe inclusions are formed. Additional alloying of alloys with nickel and manganese contributes to the appearance of these inclusions at a lower iron content. Iron has a modifying effect on the structure of aluminum bronzes, increases their strength, hardness and anti-friction properties, and reduces the tendency to embrittlement of two-phase bronzes.
Aluminum-iron bronzes (for example, BrAZh9-4) have the best ductility after normalization at 600...700°C or hardening from 950°C followed by tempering at 250...300°C.
Nickel contributes to additional strengthening of bronzes alloyed with iron and nickel due to aging. For example, in the annealed (soft) state of BrAZHN10-4-4, the hardness is 160 HB. After hardening from 980°C and aging at 400°C for 2 hours, the hardness increases to 400 HB.
Aluminum-iron-nickel bronzes are used to make parts that operate under severe wear conditions at elevated temperatures (400...500°C): valve seats, exhaust valve guides, parts of pumps and turbines, gears, etc.
Aluminum-iron bronzes alloyed with cheaper manganese instead of nickel (BrAZhMts10-3-1.5) have high mechanical, anti-corrosion and technological properties.
4.5.3.3 Silicon bronzes
Silicon bronzes contain up to 3% Si and have a single-phase α-solid solution structure. The single-phase structure of the solid solution provides silicon bronzes with high ductility and good workability under pressure. When the silicon content increases to more than 3%, a hard and brittle γ-phase appears in the structure of the alloys, which reduces their ductility.
Additions of manganese and nickel increase the strength and hardness of silicon bronzes. Nickel, having variable solubility in the α-phase, creates the opportunity to strengthen nickel-silicon bronzes by hardening and aging. After quenching from 800°C and aging at 500°C, BrKN-1-3 and BrKN-0.5-2 have σв > 700 MPa, δ ≈ 8%.
Silicon bronzes are produced in the form of tapes, strips, rods, and wires. They are rarely used for shaped castings. They are used instead of more expensive tin bronzes in the manufacture of antifriction parts (BrKN1-3), (BrKMtsZ-1), as well as to replace beryllium bronzes in the production of springs, membranes and other parts of devices operating in fresh and sea water.
The casting properties of silicon bronzes are lower than those of tin, aluminum bronzes and brasses. Alloying with zinc helps to improve the casting properties of these bronzes
4.5.3.4 Beryllium bronzes
Beryllium bronzes are characterized by high strength and elasticity, hardness and corrosion resistance combined with increased fatigue resistance. Double beryllium bronzes contain on average 2.0...2.5% Be (BrB2, BrB2.5), since with a higher beryllium content the ductility becomes very low.
Beryllium has variable solubility in copper, which decreases with decreasing temperature, which makes it possible to carry out hardening, which consists of hardening from 780...800°C and subsequent aging at 325°C. The most common beryllium bronze BrB2 after quenching at 780°C and aging at 300...350°C for 2 hours has the following mechanical properties: σв = 1250 MPa, σ0.2 = 1000 MPa, δ = 2.5%, hardness 700 HB , E = 133 GPa. Plastic deformation of hardened bronze and subsequent aging make it possible to increase the temporary resistance to 1400 MPa.
Beryllium bronzes are heat-resistant materials that operate stably at temperatures up to 310...340°C. At 500°C they have approximately the same tensile strength as tin-phosphorus and aluminum bronzes at room temperature.
Beryllium bronzes have high thermal and electrical conductivity. They are well processed by cutting, welded by spot and roller welding, but the wide temperature range of crystallization makes their arc welding difficult.
Beryllium bronzes are produced mainly in the form of strips, tapes, wires and other deformed semi-finished products. At the same time, they can be used to produce high-quality shaped castings. Beryllium bronzes are used to make critical parts: elastic elements of precision instruments (flat springs, spring contacts, membranes); wear parts (cams, gears, worm gears); bearings operating at high speeds, high pressures and elevated temperatures.
Beryllium bronze is used to make tools that do not create a spark when hitting metal or stone, which allows it to be used in explosive mining operations.
The main disadvantage of beryllium bronzes is their high cost. Alloying with Mg, Ni, Ti, Co makes it possible to reduce the beryllium content to 1.7...1.9% without a noticeable decrease in mechanical properties (BrBNT1.7, etc.).
Copper is a metal without polymorphic transformations with a fcc crystalline lattice. The melting point is 1083°C. When heated, there are no polymorphic transformations in copper. The density of copper is 8940 kg/m3.
Copper is easily polished, soldered and welded well. In terms of electrical conductivity and thermal conductivity, copper ranks second after silver. Copper has high corrosion resistance in atmospheric conditions, fresh and sea water, caustic alkalis, organic acids and other aggressive environments, but interacts with ammonia and sulfur dioxide gases.
Copper is well rolled into thin sheets and strips. Cold plastic deformation (reaching 90% or more) increases the strength, hardness, and elastic limit of copper, but reduces ductility and electrical conductivity. During plastic deformation, a texture appears that causes anisotropy in the mechanical properties of copper. Annealing to remove hardening is carried out at 550 – 600°C in a reducing atmosphere, since copper easily oxidizes when heated.
The disadvantages of copper include: low strength, poor machinability and low fluidity.
Depending on the content of impurities, the following grades of copper are distinguished: M00 (99.99% Cu), M0 (99.97% Cu), M1 (99.9% Cu), M2 (99.7% Cu), M3 (99. 5% Cu).
The most commonly found elements in copper are divided into three groups.
The first group includes copper-soluble elements Al, Fe, Ni, Sn, Zn, which increase the strength and hardness of copper and are used for alloying copper-based alloys. These impurities sharply reduce the electrical and thermal conductivity of copper.
The second group consists of copper-insoluble elements Pb and Bi, which worsen the mechanical properties of copper and single-phase alloys based on it. Forming low-melting eutectics (at 326 and 270°C, respectively), located along the grain boundaries of the main phase, they cause red brittleness of the alloys.
Insoluble elements O, S, Se, Te are present in copper and its alloys in the form of intermediate phases (for example, Cu2O, Cu2S). They constitute the third group of elements and form eutectics with copper with a high melting point that do not cause red brittleness. Oxygen when annealing copper in hydrogen causes “hydrogen disease”, which can lead to destruction of the metal during pressure treatment or operation of finished parts.
To alloy copper alloys, elements soluble in copper are mainly used - Zn, Sn, Al, Be, Si, Mn, Ni. By increasing the strength of copper alloys, alloying elements practically do not reduce, and some of them (Zn, Sn, Al) increase ductility. High ductility is a distinctive feature of copper alloys. The relative elongation of some single-phase alloys reaches 65%. Copper alloys are inferior in strength to steel. The tensile strength of most copper alloys lies in the range of 300...500 MPa, which corresponds to the properties of low-carbon unalloyed steels in a normalized state. And only the most durable beryllium bronzes after hardening and aging have a tensile strength of 1100...1200 MPa and corresponds to the strength level of medium-carbon alloy steels subjected to thermal improvement.
Copper alloys are divided into two main groups: brass and bronze.
Copper alloys are labeled by chemical composition, using letters to designate elements and numbers to indicate their mass parts. In copper alloys, aluminum is designated by the letter A, beryllium - B, iron - F, silicon - K, magnesium - Mg, nickel - N, tin - O, lead - C, phosphorus - F, zinc - C, zirconium - Cr, chromium - X; manganese – Mts.
Brasses (alloys of copper and zinc) are marked with the letter L
.
In deformable brasses, which do not contain other elements besides copper and zinc, the letter L
is followed by a number indicating the average copper content.
In multi-component brasses, after L
there are letters - symbols of the elements, and then numbers indicating the content of copper and each alloying element.
For example, L68
contains 68% Cu,
LAN59-3-2
contains 59% Cu, 3% Al;
2% Ni (rest Zn). In grades of foundry brass, the zinc content is indicated, and the amount of each alloying element is placed directly after the letter indicating it. For example, brass LTs40Mts3A
contains 40% Zn, 3% Mn and 1% Al.
Bronzes (alloys of copper with all elements except zinc) are designated by the letters Br
, followed by letters and numbers.
In stamps of wrought bronzes, letters are first placed - symbols of alloying elements, and then numbers indicating their content. For example, BrAZh9-4
contains 9% A1, 4% Fe, the rest is Cu.
In grades of cast bronzes, after each letter the content of this alloying element is indicated. For example, BrO6Ts6S3
contains 6% Sn, 6% Zn, 3% Pb, the rest is Cu.
Brass
Copper with zinc forms an a-solid solution with a limiting zinc concentration of 39% (Fig. 22). With a higher zinc content, an electronic compound CuZn (β-phase) with a bcc crystal lattice is formed. At 454...468°C (dashed line in the diagram), ordering of the β-phase occurs, and the ordered β-solid solution is designated as the β'-phase.
Figure 22 — Cu-Zn phase diagram
If there is only an α-solid solution in the brass structure, an increase in the zinc content causes an increase in its strength and ductility. The appearance of the β'-phase is accompanied by a sharp decrease in plasticity and an increase in hardness and brittleness. Strength continues to increase as the zinc content increases to 45% while the brass is in the two-phase state. With a further increase in the percentage of zinc, brass transitions to a single-phase state with a β'-phase structure, which causes a sharp decrease in strength. Brasses containing up to 45% Zn are of practical importance. Alloys with a high zinc content are highly brittle.
Double brasses (containing only copper and zinc) are divided into single-phase (with an α-solid solution structure) and two-phase (with an α and β-phase structure).
Single-phase brasses have high ductility and respond well to cold plastic deformation, which significantly increases their strength and hardness. To increase plasticity, recrystallization annealing is carried out at 600...700°C.
Parts that are made of wrought brass with a content of more than 20% zinc may be subject to “seasonal” cracking in humid air in the presence of sulfur gases in the atmosphere. To prevent cracking, parts are annealed at temperatures below the recrystallization temperature (in most cases at 250...270°C).
Increasing the zinc content makes brass cheaper, improves its machinability and ability to resist wear, but at the same time, thermal conductivity and electrical conductivity decrease.
Impurities increase the hardness and reduce the ductility of brass. Lead and bismuth are especially unfavorable, causing red brittleness in single-phase brasses. Therefore, single-phase brasses are not subjected to hot deformation, but are produced in the form of cold-rolled strips, tapes, wires, and sheets. Rolled steel is used to produce parts using the deep drawing method (radiator tubes, shell casings, bellows, pipelines), as well as parts that require low hardness due to operating conditions (washers, bushings, O-rings, etc.).
Due to the short crystallization temperature range, double brasses have a low tendency to dendritic segregation, high fluidity, low diffuse shrinkage porosity and good tightness. But, despite this, they are practically not used for shaped castings, since they have a rather large concentrated shrinkage cavity.
To increase machinability, lead is added to brass. Brass LS59-1 (“automatic”) is supplied in rods and is intended for the manufacture of parts on automatic machines.
Al, Fe, Ni, Sn, Si are used to alloy brass. These elements increase the strength and corrosion resistance of brass. Therefore, alloyed brasses are widely used in river and marine shipbuilding (LAZH60-1-1). Brasses alloyed with tin (LO70-1, LO62-1) have high corrosion resistance in sea water and therefore are called “marine brasses”.
Practical applications are found in brass with the addition of aluminum up to 4% (LA77-2), which, due to their single-phase structure, can be easily processed under pressure.
Aluminum brasses are additionally alloyed with nickel, iron, manganese, silicon, which have variable solubility in α-solid solution, which allows these brasses to be strengthened by hardening and aging.
Brass LANKMts75-2-2.5-0.5-0.5 is the only alloy based on the Cu-Zn system, which is strengthened by dispersion hardening. The hardening temperature is 800°C, after which brass has high ductility, and after aging it acquires high strength (sв = 700 MPa, d = 25%). Good ductility in the hardened state allows the alloys to be further strengthened by plastic deformation before aging, which ensures an increase in tensile strength up to 1000 MPa.
Silicon brasses are characterized by high strength, ductility, and toughness both at normal and low temperatures (up to -183°C). When alloying brasses, small additions of silicon (LK80-3) are used to obtain a single-phase structure. Such brasses are used for the manufacture of fittings, instrument parts, in shipbuilding and mechanical engineering.
Bronze
4.5.3.1 Tin bronzes
The limiting solubility of tin in copper corresponds to 15.8%, but at a tin concentration of more than 10%, a δ-phase is formed in the structure of bronzes, causing a sharp decrease in their viscosity and ductility. Therefore, bronzes containing only up to 10% Sn are of practical importance.
Among copper alloys, tin bronzes have the lowest linear shrinkage, which makes it possible to produce complex shaped castings. Double and low alloy casting bronzes contain 10% Sn. Tin bronzes are alloyed with Zn, Pb, P (BrO3Ts12S5, BrO4Ts4S17, BrO10Ts2, etc.). Zinc completely dissolves in an α-solid solution when alloyed to 15% and, by reducing the crystallization interval of tin bronzes, improves their fluidity and density of castings. Lead increases the anti-friction properties and improves the machinability of tin bronzes. Phosphorus, being a deoxidizer of tin bronzes, increases their fluidity, and wear resistance improves due to the appearance of solid inclusions of copper phosphide Cu3P.
High corrosion resistance in atmospheric conditions, fresh and sea water contributes to the widespread use of casting bronzes for steam-water fittings operating under pressure.
Deformable bronzes contain up to 6...8% Sn (BrOF 4-2.5, BrOTs 4-3, etc.). To eliminate dendritic segregation and level out the chemical composition, as well as improve workability under pressure, diffusion annealing is used, which is carried out at 700...750°C. During cold plastic deformation, bronze is subjected to intermediate annealing at 550...700°C. Wrought bronzes are characterized by good ductility and higher strength than cast bronzes.
Deformable bronzes have high elastic properties and fatigue resistance. They are used for the manufacture of round and flat springs in precision mechanics, electrical engineering, chemical engineering and other areas of industry.
4.5.3.2 Aluminum bronzes
Aluminum bronzes are distinguished by high mechanical, anti-corrosion and anti-friction properties. Their advantages over tin bronzes are lower cost and higher mechanical and technological properties. In particular, a short crystallization interval provides aluminum bronzes with high fluidity, concentrated shrinkage and good tightness of castings, and a low tendency to dendritic segregation.
Copper and aluminum form an α-solid solution. With an increase in aluminum content to 4.5%, along with strength and hardness, ductility increases, which then drops sharply, and strength continues to increase with an increase in aluminum content to 10...11%. With the appearance of a eutectoid containing hard and brittle phases at these concentrations, wear resistance increases and antifriction properties appear.
Single-phase bronzes (BrA5, BrA7) are deformable. They have the best combination of strength (σв = 400...450 MPa) and ductility (δ = 60%).
Two-phase bronzes are distinguished by high strength (σв = 600 MPa) and hardness (> 100 HB). They can be subjected to strengthening heat treatment.
Aluminum bronzes are alloyed with iron, nickel, and manganese. In the α-phase of aluminum bronze, up to 4% iron is dissolved; at higher contents, Al3Fe inclusions are formed. Additional alloying of alloys with nickel and manganese contributes to the appearance of these inclusions at a lower iron content. Iron has a modifying effect on the structure of aluminum bronzes, increases their strength, hardness and anti-friction properties, and reduces the tendency to embrittlement of two-phase bronzes.
Aluminum-iron bronzes (for example, BrAZh9-4) have the best ductility after normalization at 600...700°C or hardening from 950°C followed by tempering at 250...300°C.
Nickel contributes to additional strengthening of bronzes alloyed with iron and nickel due to aging. For example, in the annealed (soft) state of BrAZHN10-4-4, the hardness is 160 HB. After hardening from 980°C and aging at 400°C for 2 hours, the hardness increases to 400 HB.
Aluminum-iron-nickel bronzes are used to make parts that operate under severe wear conditions at elevated temperatures (400...500°C): valve seats, exhaust valve guides, parts of pumps and turbines, gears, etc.
Aluminum-iron bronzes alloyed with cheaper manganese instead of nickel (BrAZhMts10-3-1.5) have high mechanical, anti-corrosion and technological properties.
4.5.3.3 Silicon bronzes
Silicon bronzes contain up to 3% Si and have a single-phase α-solid solution structure. The single-phase structure of the solid solution provides silicon bronzes with high ductility and good workability under pressure. When the silicon content increases to more than 3%, a hard and brittle γ-phase appears in the structure of the alloys, which reduces their ductility.
Additions of manganese and nickel increase the strength and hardness of silicon bronzes. Nickel, having variable solubility in the α-phase, creates the opportunity to strengthen nickel-silicon bronzes by hardening and aging. After quenching from 800°C and aging at 500°C, BrKN-1-3 and BrKN-0.5-2 have σв > 700 MPa, δ ≈ 8%.
Silicon bronzes are produced in the form of tapes, strips, rods, and wires. They are rarely used for shaped castings. They are used instead of more expensive tin bronzes in the manufacture of antifriction parts (BrKN1-3), (BrKMtsZ-1), as well as to replace beryllium bronzes in the production of springs, membranes and other parts of devices operating in fresh and sea water.
The casting properties of silicon bronzes are lower than those of tin, aluminum bronzes and brasses. Alloying with zinc helps to improve the casting properties of these bronzes
4.5.3.4 Beryllium bronzes
Beryllium bronzes are characterized by high strength and elasticity, hardness and corrosion resistance combined with increased fatigue resistance. Double beryllium bronzes contain on average 2.0...2.5% Be (BrB2, BrB2.5), since with a higher beryllium content the ductility becomes very low.
Beryllium has variable solubility in copper, which decreases with decreasing temperature, which makes it possible to carry out hardening, which consists of hardening from 780...800°C and subsequent aging at 325°C. The most common beryllium bronze BrB2 after quenching at 780°C and aging at 300...350°C for 2 hours has the following mechanical properties: σв = 1250 MPa, σ0.2 = 1000 MPa, δ = 2.5%, hardness 700 HB , E = 133 GPa. Plastic deformation of hardened bronze and subsequent aging make it possible to increase the temporary resistance to 1400 MPa.
Beryllium bronzes are heat-resistant materials that operate stably at temperatures up to 310...340°C. At 500°C they have approximately the same tensile strength as tin-phosphorus and aluminum bronzes at room temperature.
Beryllium bronzes have high thermal and electrical conductivity. They are well processed by cutting, welded by spot and roller welding, but the wide temperature range of crystallization makes their arc welding difficult.
Beryllium bronzes are produced mainly in the form of strips, tapes, wires and other deformed semi-finished products. At the same time, they can be used to produce high-quality shaped castings. Beryllium bronzes are used to make critical parts: elastic elements of precision instruments (flat springs, spring contacts, membranes); wear parts (cams, gears, worm gears); bearings operating at high speeds, high pressures and elevated temperatures.
Beryllium bronze is used to make tools that do not create a spark when hitting metal or stone, which allows it to be used in explosive mining operations.
The main disadvantage of beryllium bronzes is their high cost. Alloying with Mg, Ni, Ti, Co makes it possible to reduce the beryllium content to 1.7...1.9% without a noticeable decrease in mechanical properties (BrBNT1.7, etc.).