Argon is one of the most common chemical elements present in the atmosphere of planet Earth, ranking third after nitrogen and oxygen. At the same time, obtaining argon is a responsible and complex process from a technological point of view, which only real professionals can handle. Its peculiarity lies in the fact that in industry this inert gas is obtained by separating air into nitrogen and O2.
But there is also a certain difficulty - the boiling point of this substance is between the boiling temperatures of these two gases. To be more precise, it differs by less than 3 degrees from the value at which O2 boils. Because of this, it is incredibly difficult to separate the two fractions using the rectification method. After all, Ar will simply be distributed among other substances-components of the air, joining oxygen in most cases.
But the widespread distribution of Ar and its demand in a variety of fields of activity have forced humanity to develop more and more new methods and technological methods to make the process of producing argon simpler, more convenient and more effective. Moreover, many of them are associated with the production of industrial gases that have undergone deep purification (you can read our separate material on the topic of especially pure gaseous substances).
History of discovery
The background to the discovery of Ar began in 1785. An outstanding scientist and naturalist from Great Britain, Henry Cavendish, studied the composition of air. He subjected nitrogen to oxidation and weighed the resulting oxides. At the end of the experiment, gas remained in the vessel. Cavendish determined its volume to be 0.8% of the initial volume of air.
The scientist was unable to determine the composition of this gas. A century later, Sirs John Rayleigh and William Ramsay returned to the problem. During their experiments, they discovered that nitrogen released from the air has a higher density than nitrogen obtained during the decomposition reaction of ammonium nitrite.
in 1884 they managed to isolate a certain gas from the air that was denser than nitrogen. This substance had a monatomic molecular structure and was extremely inert - i.e. did not react with other substances.
At a meeting of the Royal Society, the new gas was given the name “argon”, which translated from ancient Greek meant “calm, lazy”
Argon in nature
Due to its almost complete inertness, Ar is present in the natural environment exclusively in unbound form. Its percentage in different parts of the Earth is approximately:
- earth's crust - 0.00012%;
- sea water - 0.00045%;
- atmosphere - 0.926%.
The proportion of Ar in the air is higher than the total proportion of all other inert gases. The main source for its production is our atmosphere.
Content of gases in the atmosphere
Argon is also contained in the Earth's crust in the form of the radioactive isotope Argon-40 and appears during the decay reaction of Potassium isotopes.
Modern science, along with other inert gaseous elements, classifies Ar into group VIII of the periodic table.
Isotopes
Argon is represented in the earth's atmosphere by three stable isotopes: 36Ar (0.337%), 38Ar (0.063%), 40Ar (99.600%). Almost the entire mass of the heavy isotope 40Ar arose on Earth as a result of the decay of the radioactive isotope of potassium 40K (the content of this isotope in igneous rocks averages 3.1 g/t). The decay of radioactive potassium occurs in two directions simultaneously:
The first process (ordinary β-decay) occurs in 88% of cases and leads to the formation of a stable isotope of calcium. In the second process, where 12% of the atoms participate, electron capture occurs, resulting in the formation of a heavy isotope of argon. One ton of potassium contained in rocks or waters generates approximately 3,100 argon atoms over the course of a year. Thus, 40Ar gradually accumulates in minerals containing potassium, which makes it possible to measure the age of rocks; The potassium-argon method is one of the main methods of nuclear geochronology. The probable sources of origin of the 36Ar and 38Ar isotopes are unstable products of the spontaneous fission of heavy nuclei, as well as the reaction of capture of neutrons and alpha particles by the nuclei of light elements contained in uranium-thorium minerals. The overwhelming majority of cosmic argon consists of the isotopes 36Ar and 38Ar. This is due to the fact that potassium is approximately 50,000 times less abundant in space than argon (on Earth, potassium predominates over argon by 660 times). The calculation made by geochemists is noteworthy: by subtracting radiogenic 40Ar from the argon of the earth's atmosphere, they obtained an isotopic composition very close to the composition of cosmic argon.
How is argon produced?
Due to the industrially significant content of argon in the air, it is obtained as an additional product of the cryogenic distillation of O2 and N2.
The technology is based on the fact that the boiling (or liquefaction) point of Ar lies between the temperatures of N2 and O2.
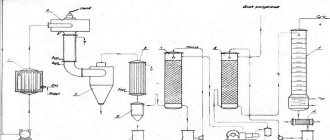
Before the process begins, the air is thoroughly cleaned of dust in multi-stage filters, dried from water vapor, and then compressed by powerful compressors until it turns into a liquid state. The liquid is distilled in a distillation column to separate it into its individual substances.
Installation for argon production
Nitrogen is the first to evaporate at -195 °C; its vapors are collected on the appropriate rectifier plate and discharged into a separate tank. The next highest (and at a boiling point of -185 °C) is the argon fraction, containing 12% Ar, less than half a percent nitrogen and oxygen. It is fed into the next distillation column, in which the percentage of Ar is brought to 85, the remainder being oxygen with traces of nitrogen. This substance is called raw argon, the starting material for producing purified gas.
Several methods are used in industry to purify raw argon from impurities.
Hydrogen added to the raw material is oxidized by a catalyst and heated to 500 °C, thus removing oxygen from the mixture. The water vapor formed on the catalyst is removed using a moisture separator. The gas is then dried. Argon with the nitrogen remaining in it is rectified again.
Alternative methods for obtaining Ar are also used. During the synthesis of ammonia from nitrogen and hydrogen in chemical reactors, Ar is obtained as a by-product. The technological component of this synthesis - purge gas - contains up to 20% Ar. The calmest element is extracted from this gas. The production cost, which consists mainly of the costs of cooling and heating the components, is divided between ammonia and argon, and is significantly lower.
The quality of the gas obtained by any method is determined by the technology for purifying it from small amounts of residual N2, O2, water vapor and H2.
A device that receives argon ion beams
Argon production: technology features and application of technical gas
Argon is one of the most common chemical elements present in the atmosphere of planet Earth, ranking third after nitrogen and oxygen. At the same time, obtaining argon is a responsible and complex process from a technological point of view, which only real professionals can handle. Its peculiarity lies in the fact that in industry this inert gas is obtained by separating air into nitrogen and O2.
But there is also a certain difficulty - the boiling point of this substance is between the boiling temperatures of these two gases. To be more precise, it differs by less than 3 degrees from the value at which O2 boils. Because of this, it is incredibly difficult to separate the two fractions using the rectification method. After all, Ar will simply be distributed among other substances-components of the air, joining oxygen in most cases.
But the widespread distribution of Ar and its demand in a variety of fields of activity have forced humanity to develop more and more new methods and technological methods to make the process of producing argon simpler, more convenient and more effective. Moreover, many of them are associated with the production of industrial gases that have undergone deep purification (you can read our separate material on the topic of especially pure gaseous substances).
What is argon?
It is a monatomic inert gas that does not have any odor, color or taste. It is one of the main components of air and is also found in large quantities in the atmosphere of our planet. Its boiling point is minus 185.9 degrees Celsius, which is very close to the boiling point of O2. It should be noted that, being in the air in high concentrations, it can be dangerous for humans, as it displaces oxygen and leads to symptoms of oxygen starvation.
According to state standards (GOST), gaseous Ar must be stored and transported in gas cylinders specially designed for this purpose, made using a steel alloy. The pure substance must be in containers that are appropriately colored and marked - a gray cylinder with a green inscription: “Pure argon”.
In what areas is it used?
Every year Ar becomes more and more popular and in demand. Inert gas, unique in its properties, is used in various fields of human activity, which include:
- Use of laser equipment – it is a mandatory element of the laser system.
- Production of light sources. First of all, these include classic incandescent lamps, the internal space of which is filled with Ar (as a result, this makes it possible to maximize the performance characteristics of such a light bulb).
- Production of plastic windows. It is used to fill the space inside a double-glazed window, which significantly increases the thermal insulation qualities of the finished window structure.
- Operation of gas systems for extinguishing fires. Monatomic gas is one of the main components used in fire extinguishing systems (you can read more about the use of gas fire extinguishing systems here).
- Application in the food industry. Here it is found as a common food additive, as well as in the creation of special environments or packaging to extend the life of various food products.
Separately, it should be noted argon welding - a technology that allows you to carry out work on welding various metals and non-metals. In this case, Ar acts as the main component for creating a special protective environment, including when using arc, resistance or laser welding. Automotive service employees may be interested in our article, which is devoted to welding alloy wheels using argon welding.
How is argon produced industrially?
Answering the question of how argon is produced, it should be noted that this is a rather difficult task. Most often, low-temperature air rectification technology is used for this, during which the air is separated into different fractions - O2 and nitrogen. In this case, Ar itself is extracted as a by-product.
adsorption unit
The essence of the technology is that during rectification, Ar volatilizes and rises up the column after nitrogen. Under the influence of low temperatures, it turns into condensate and falls down. After this, the so-called argon fraction is removed from the apparatus. This is a mixture, the main part of which is oxygen, while the percentage of Ar in it usually does not exceed 12% (also such a fraction can contain up to 0.5% nitrogen).
generator
Then, in the process of obtaining argon, the fraction is again subjected to rectification using a similar technology, resulting in “raw argon” being obtained. When in vapor form, it can contain from 75% to 95% pure substance. After this, the crude product is purified by introducing h3 and a special catalyst into it, as a result of which the oxygen contained in the mixture is spent on the oxidation of hydrogen.
separation plant diagram
Domestic manufacturers usually offer Ar, in which the content of pure substance varies from 99.9% to 99.99%. At the same time, there is also a particularly pure gas, the volume of impurities in which is very small - no more than 0.005% helium and 0.001% O2.
Conclusions about the use and production of argon
Ar is one of the most common gases, which at the same time is very difficult to obtain in its pure form. It is mined in several ways, and subsequently used in various fields of human activity, including in production, during welding work or in laser installations.
If you want to buy highly purified Ar, then you can contact. To do this, you need to follow the link https://www.propangaz.ru/?id=20. The company’s specialists will not only tell you how argon is produced, but will also offer the most favorable price for it.
General characteristics of Ar
Ar belongs to the group of inert gases. The charge of its nucleus is 18; the element is located under the same number in the periodic table.
Of all the members of group VIIIA, it is the most commonly found in nature. The volume fraction of Ar in the atmosphere is 0.93%, the mass fraction is 1.28%. The element is a colorless, tasteless and odorless gas. Chemically inactive - argon does not react and practically does not combine with any elements or substances, with the exception of CU(Ar)O and argon hydrofluoride.
Very poorly soluble in water, slightly greater solubility is observed when interacting with organic solvents.
Types of argon
When talking about types or varieties of Ar, we must understand that these are the same chemical substance. Types differ in the degree of purification from impurities.
- Top grade. Ar content is not less than 99.99%. This grade of especially high purity is used for critical welding work, such as welding materials that are chemically active in a heated state: some non-ferrous alloys, primarily titanium, stainless steel, etc. It is also used for welding highly loaded structural steel products.
- First grade. Ar content not less than 99.98%, used for welding aluminum-based alloys with other metals and alloys, for less active non-ferrous metals.
- Second grade. Ar content not less than 99.95%. Used for welding parts made of heat-resistant steel alloys, aluminum and structural steels. The use of pure Ar in these cases is undesirable, since it leads to increased porosity of the weld material and does not protect the weld pool from high humidity and other contaminants. To avoid the occurrence of such a defect, carbon dioxide and oxygen are added to the mixture of protective gases, which bind the hydrogen and other impurities released during welding. The slags formed during these reactions float to the surface of the weld pool and, after solidification, are removed along with the scale.
Areas of application of argon welding
What is argon welding used for? It is necessary in cases where welding seams must be performed flawlessly. It is especially often used to connect difficult-to-weld materials and workpieces with thin walls. This type of welding is in demand in aircraft and rocket manufacturing, and the automotive industry. Through this connection, important components are made from aluminum and its alloys.
Most often, argon arc welding is used when working with aluminum, which is difficult to weld, often cracks, and shrinks severely. In addition, in the molten state, this metal easily oxidizes, becoming covered with a refractory film, which prevents the formation of a seam. And only welding in an argon environment will help to obtain high-quality seams.
Such welding is especially in demand at automobile service stations, where with the help of this joining method the service life of parts is significantly extended.
Why is argon welding used at car service stations? It can be used to repair radiators, various parts of gearboxes, air conditioner pipes and other elements made of aluminum and its alloys. Soldering and plasma spraying, as well as other welding methods, could not be used for such work, since the parts have technical features.
Physical and chemical properties
The properties of argon are typical of a member of group VIII.
At ordinary temperatures, Ar is in a gaseous state. The molecule includes a single atom, the chemical formula is very simple: Ar. The boiling point is very low: -185.8 °C at atmospheric pressure.
Solubility in water is low - only 3.29 ml per 100 ml of liquid
The density of argon under normal conditions is 1.78 kg/m3. The molar heat capacity of the gas is 20.7 J/Kmol.
Characteristics of argon and other inert gases
The gas is almost completely inert. To date, scientists have managed to obtain only two of its compounds - CU(Ar)O and argon hydrofluoride. The compounds exist only at ultra-low temperatures. It is assumed that Ar may be part of excimer-type molecules that are unstable in the normal state. Such molecules can only exist in an excited state, for example, during a high-intensity electrical discharge. Such compounds are possible with mercury, oxygen and fluorine.
Electronegativity on the Pauling scale is 4.3.
Both the oxidation state and the electrode potential have a zero value, which is typical for an inert gas.
The ionic radius is 154, the covalence radius is 106 PM. Ionization threshold - 1519 kJ/mol
Atomic and molecular mass
Such important parameters as atomic and molecular masses show how much the mass of a molecule of a substance and the mass of its atom, respectively, exceed a value equal to one twelfth of the mass of a hydrogen atom.
Due to the fact that the Ar molecule consists of a single atom, the molecular and atomic mass of argon are identical and amount to 39.984.
Argon structure and properties
Isotopes
Under natural conditions, Ar occurs as three stable isotopes
- 36Ar – the percentage of this isotope is 0.337% in the nucleus of 18 protons and 18 neutrons;
- 38Ar - its share is only 0.063%, there are 18 protons and 20 neutrons in the nucleus;
- 40Ar is the most common, its share is 99.6%, the nucleus also has 18 protons, but already 22 neutrons.
It was possible to artificially obtain isotopes with a mass index from 32 to 55, the most stable of which was 39Ar, whose half-life is 268 years.
The large percentage of 40Ar among the isotopes found in nature is caused by its constant formation during the decay reaction of the potassium-40 isotope. Per 1000 kg of potassium during such reactions no more than 3100 40Ar atoms are formed per year. But, since these reactions take place continuously over hundreds of millions of years, the isotope has accumulated in nature in significant volumes.
The dominance of the heavy isotope in nature determines the fact that the atomic weight of Ar exceeds the atomic weight of potassium, which is located next to it in the table. When the Periodic Table was created, there was no such contradiction, since argon was discovered and its properties were studied much later, in the first decade of the 20th century. Ar was initially placed in the first group of the table; the eighth group was allocated later.
Ions
Like other noble gases (such as He and Ne), Ar is susceptible to ionization. When atoms are excited and given high energies, molecular Ar2+ ions appear.
Molecule and atom
For inert gases, these concepts are identical, since these elements do not want to enter into a chemical bond even with their own kind. The molecule includes one atom, the chemical formula of the gas does not differ from the designation of the element: Ar.
Molar mass
The molar mass of argon is 39.95 g/mol.
There are several methods for calculating it:
- Using the relative atomic mass M and the proportionality coefficient k, expressing the relationship between the relative mass and the molar mass. This coefficient is a universal constant and is equal for all elements. Molar mass M is expressed as the product of the proportionality coefficient and the relative mass.
- Using molar volume. You will need to find the volume occupied by a certain mass of gas under normal conditions, then calculate the mass of 22.4 liters of the substance under the same conditions.
- Using the Mendeleev-Clapeyron equation simulating an ideal gas.
pV = mRT / M,
Having carried out the transformations, we obtain the expression for the molar mass:
M=mRT/pV
Where
- p – pressure in pascals,
- V – volume in cubic meters
- m – mass in grams,
- T - temperature in Kelvin,
- R is a constant whose value is 8.314 J/(mol×K).
Application area
Argon is most widely used for welding. It is used to create a protective atmosphere around the weld pool, displacing O2 and N2 contained in the atmosphere from the working area. This is especially important for welding non-ferrous metals, many of which, for example, Ti, are characterized by high chemical activity when heated. Inert gas is also indispensable for permanent connections of stainless and high-alloy alloys.
It is also widely used in the installation of highly loaded building structures, such as frames of high-rise buildings, bridge trusses and many others. Here its use ensures high quality, uniformity and durability of critical connections. In the construction industry, argon welding dominates among other methods.
Argon welding
Argon arc welding
Argon welding is no less widely used in mechanical engineering, primarily in the chemical and food industries. The seams are durable and reliable, even when exposed to aggressive environments.
The oil and gas industries also use argon welding in the installation of pipelines, gas pumping stations and oil refineries.
The method is also used in the nuclear industry, transport engineering and the aerospace industry.
In households, argon welding is not so widespread. This is explained:
- high cost of equipment and consumables;
- the need for sufficient qualification of the welder;
- lower loads experienced by home structures;
- lower requirements for the strength and durability of welded joints.
If a household has an occasional need for such welding work, then it is cheaper, faster and more reliable to invite a specialist welder.
Double-glazed window with argon
The principle of operation of a double-glazed window with argon
A characteristic property of Ar is its higher density compared to air. Therefore, the maximum efficiency of argon welding is achieved in the lower welding position. In this case, the inert substance spreads over the surface of the part and forms a protective cloud of considerable extent, allowing welding to be carried out both with high currents and at high speed. When welding in an inclined and upper position, it is necessary to take into account the “falling through” of argon through the air. To compensate for this phenomenon, either increase the gas supply or carry out work in a sealed room filled with inert gas. In both cases, the cost of work increases.
Since the ionization potential of Ar is low, its use ensures ideal geometric characteristics of the weld, especially the profile. An excited electric arc in an argon atmosphere is also characterized by high stability of its parameters. On the other hand, a low ionization potential also causes a lower arc ignition and maintenance voltage. This reduces its heat generation and complicates the penetration of thick sheets of metal.
The higher arc temperature in an argon atmosphere significantly increases weld penetration. This allows welding to be carried out in one pass, provided that the parameters of the gap between the workpieces are strictly observed.
When using the TIG welding method, the argon atmosphere protects not only the welding zone, but also the end of the infusible electrode from corrosive influences.
In a number of specific cases, helium is added to the protective gas mixture.
In addition to being used for welding, argon is used:
- As a plasma-forming substance in installations for plasma cutting of metal.
- To create an inert environment in food packaging. It displaces air oxygen and water vapor from bags and containers, which adversely affect the shelf life of products. Products in a protective atmosphere are stored several times longer than in conventional packaging. This method is also used for packaging medical products and drugs, allowing them to be kept in proper sterility and chemical purity.
- As an active agent in fire extinguishing installations. Argon displaces oxygen (or other gas) from the combustion site, stopping it.
- To create a protective environment in technological installations when processing semiconductor devices, creating microcircuits and other electronic components or materials of high purity levels.
- Filler for electric lamps.
- In advertising fluorescent tubes.
Types of equipment for argon arc welding
There are several types of argon welding, which depend on the level of mechanization of the process:
- manual;
- mechanized;
- automated;
- robotic.
Each welding method requires certain equipment, and accordingly, the cost of the work varies.
In the manual welding method, non-consumable tungsten electrodes are used, the wire is fed by the master himself, and the torch for argon arc welding moves.
The mechanized method is characterized by the supply of filler rods in automatic mode, while the master himself holds the torch.
Automated welding is completely under operator control - wire feeding and torch movement occur automatically.
The robotic process also eliminates the presence of an operator.
Dependence of argon pressure in a cylinder on temperature
As it heats up, the pressure of the gaseous substance in a closed volume increases. The table shows approximate pressure values in the cylinder depending on the ambient temperature.
| T, °C | P, Megapascal |
| -40 | 10,45 |
| -30 | 11,33 |
| -20 | 12,21 |
| -10 | 12,92 |
| 0 | 13,74 |
| +10 | 14,62 |
| +20 | 15,33 |
| +30 | 16,03 |
It should be borne in mind that the balloon pressure does not change instantly, but as it warms up or cools down.