Content
The same substance can be in three different states of aggregation depending on conditions. For example, ice, water and water vapor (Figure 1).
Figure 1. Aggregate states of one substance using the example of ice, water and steam
Accordingly, it is one substance in solid, liquid and gaseous states. These states differ from each other in location, nature of movement and interaction of molecules . In liquids and solids, unlike gases, molecules cannot move far away from each other. Initially, they are located close to each other and their average kinetic energy is insufficient to do the work to overcome the forces of molecular attraction.
However, in practice we often observe how bodies pass from a solid to a liquid state, and vice versa. For example, the process of ice melting or freezing. In this lesson we will take a closer look at these processes and find out under what conditions they occur.
Description of metal
Tin is a rare metal, found in small quantities in ores and sands, and is especially common in layers raised from the ocean floor. It ranks 47th in abundance among metals in the earth's crust.
To obtain it, ores are used whose content of this metal is about 0.1%. First, the ore is enriched (by magnetic separation or gravity flotation). Thus, the tin content increases to 40–70% . Then the concentrate is fired in oxygen to remove arsenic and sulfur impurities. The material thus obtained is reduced in electric furnaces using aluminum or coal. The following types of this material are produced: tin in pigs, in rods, in wire. And also tin: food grade and for tinning the body.
About 40% of the world's tin production goes into the production of tin cans. The rest is used in metallurgy to produce various alloys. The most famous alloy is bronze, consisting of tin and copper. And only 7% of tin produced in the world is used in the form of chemical compounds.
Melting and melting point
- If we impart enough energy to a body, it is possible to transform it from a solid to a liquid (melting ice) and from a liquid to a gas (turning water into steam)
- If a body gives off energy, it can go from a gaseous state to a liquid state and from a liquid to a solid state.
Melting is the transition of a substance from a solid to a liquid state.
In order for the melting of a body to begin, it must be heated to a certain temperature.
The melting point of a substance is the temperature at which a substance melts.
Different substances melt at different temperatures. Ice will begin to melt if we take it in our hand, and to melt iron we will need a special furnace. A piece of tin or lead can be melted in a steel spoon.
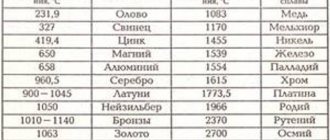
Table 1 shows the melting points of various substances. You may notice that their range is very wide.
| Substance | $t_{pl},\degree C$ | Substance | $t_{pl},\degree C$ |
| Hydrogen | -259 | Zinc | 420 |
| Oxygen | -219 | Aluminum | 660 |
| Nitrogen | -210 | Silver | 962 |
| Alcohol | -114 | Brass | 1000 |
| Mercury | -39 | Gold | 1064 |
| Ice | 0 | Copper | 1085 |
| Cesium | 29 | Cast iron | 1200 |
| Potassium | 63 | Steel | 1500 |
| Sodium | 98 | Iron | 1539 |
| Tin | 232 | Platinum | 1772 |
| Lead | 327 | Osmium | 3045 |
| Amber | 360 | Tungsten | 3387 |
Table 1. Melting points of some substances (at normal atmospheric pressure)
MELTING
MELTING, the transition of a substance from a solid crystalline. state into liquid; phase transition of the 1st order, accompanied by an abrupt change in the volume and entropy of the substance. At constant pressure, melting of single-component substances occurs at a certain fixed temperature Tmelting, called the melting temperature. This property distinguishes crystalline. substances from amorphous ones, the transition of which to the liquid state occurs gradually over a certain temperature range. As a result of P., positional disorder of the system occurs: the regular spatial arrangement of atoms or molecules (long-range order) is replaced by an irregular one, and cf. the distances between particles change slightly. In a number of molecular crystals, other mechanisms of disorder during transformation are also distinguished (orientational, configurational, vibrational). The entropy of a substance during P. increases, and depending on the nature of the change in the structure of the substance, certain mechanisms of disorder make a difference. contribution to its growth. For example, for semiconductors that transform into metallic materials during transition. state, a significant contribution to entropy is due to an increase in the concentration of conduction electrons during P. To break the bonds between particles during P., energy is required, the value of which depends on the specific substance and is called the heat of fusion.
P. temperatures of different substances lie in a wide range. Among single-component substances, hydrogen has the lowest temperature at atmospheric pressure (Tm = 14 K); the most refractory metal is tungsten (Tm = 3693 K), and the most refractory compounds are carbides, for example. TaC (Tm=4258 K) and HfC (Tm=4163 K).
Rice. 1. Phase diagram of a single-component substance; A – triple point; K – critical point.
In the phase diagram of a single-component substance (Fig. 1), the dependence of Tm on pressure (line P.) is described by line AB, corresponding to the phase equilibrium between the crystal and the liquid. The AD line (the continuation of the P line into the region of negative pressures) describes the metastable state of equilibrium of a crystal with a liquid in an extended state. The course of the P line is determined by the sign of the change in volume. For most substances, an increase in volume with P. and an unlimited increase in Tm with increasing pressure are observed (the normal course of the P. line). This means higher order and crystalline density. phase compared to the melt. At the same time, there are known substances (for example, gallium, bismuth, ice) in which, at relatively low pressures, a decrease in volume during P. and a decrease in Tm with increasing pressure are observed (an anomalous course of the P. line). When P. of such substances forms a disordered structure with a denser packing of particles. However, with increasing pressure, these substances undergo a polymorphic transition, after which the course of the P. line becomes normal. On the P. lines of some substances (for example, cesium, barium) a temperature maximum is observed, beyond which (with increasing pressure) the course of the P. line becomes anomalous, and at the maximum point the crystal and the melt have the same density. With a further increase in pressure, such substances undergo a polymorphic transition, after which the slope of the P curves again becomes positive.
On the P line (in contrast to the liquid-vapor equilibrium line) there are no features such as a critical point, which is associated with the difference in the symmetry of the crystal and the liquid. The phase diagram of helium has a special form: it does not have the triple equilibrium point crystal–liquid–vapor. Solid helium can only exist at elevated pressure, which at 0 K for 4He is approx. 2.5 MPa, for 3He – approx. 3.4 MPa.
When P. there is a size effect, which is noticeably manifested in samples of submicron size. In such crystals, a large proportion of atoms are located in the surface layer. This leads to the fact that the transition from crystalline. state to liquid, associated with a decrease in thermodynamic energy. system occurs at a lower temperature than in massive samples. For example, if for macroscopic lead sample Tm = 600 K, then for samples with sizes of 10, 5 and 3 nm Tm is 580, 540 and 490 K, respectively.
Rice. 2. Phase diagram of a two-component system.
For multicomponent systems, Tm depends on their composition. Thus, for a two-component system with unlimited solubility of the components, the dependence of Tm on the concentration x of one of them at constant pressure and the same crystalline symmetry. the gratings have the form shown in Fig. 2. The concentrations of components in the crystal and melt that are in equilibrium are different (xcr and xl in Fig. 2 at temperature T1). Lines S and L in the diagram describe, respectively, the dependence of the beginning temperature (solidus curve) and the end temperature (liquidus curve) of the liquid on concentration and limit the region in which crystals of the solid solution and the melt coexist. For the case of limited solubility of components (with different symmetries of crystal lattices), the phase diagram has a more complex form.
P. plays an important role in nature and human life (P. ice and snow, processes in the bowels of the Earth and space, etc.). P. is a component of the plural. technological processes (production of pure metals and alloys, products made from them).
The concept of specific heat of crystallization
The specific heat of crystallization (melting) is understood as the amount of energy released (consumed) by 1 kg. substances during the transition from liquid to solid (and vice versa). It is important to note that during the process of crystallization (melting), the temperature of the substance does not change and it has already been brought to a value at which the process itself is possible.
The specific heat of crystallization (melting) is measured in J/kg, denoted by the letter of the Greek alphabet λ. A-priory:
where Q is the amount of energy released (consumed) by m kilograms of the substance.
Alloys and some features of tin
The use of tin alloys is common as antifriction materials, that is, materials that have a low coefficient of friction, or that can reduce the coefficient of friction of other materials. Using antifriction materials, you can significantly increase the life of mechanisms and machines by reducing friction losses.
Another interesting feature of tin (the so-called white) is its ability to increase its volume by 25.6% when the temperature drops to 13.2°C . In this case, the so-called gray tin is formed. When the temperature reaches -33°C, the metal cracks and becomes powder. If gray and white metal come into contact, “contamination” of the white will occur. The combination of such phenomena is called the “tin plague.”
Various tin-based alloys are used in electrical engineering in the manufacture of electrical capacitors. In capacitors, staniol is actively used: almost pure metal in the form of thin sheets.
Types of metal alloys
The types of metal alloys vary based on their melting point, so the alloy variants are as follows:
- Low-melting (tin, zinc, lead, bismuth) with a melting point of no more than 600 °C.
- Medium melting (aluminum, magnesium, nickel, iron) with a temperature of 600 - 1,600 °C.
- Refractory (molybdenum, tungsten, titanium) with a temperature of more than 1,600 °C.
Next, we’ll tell you a little about the types of steels, wood alloy and solders.
Features of Carbon Steel
This material contains an admixture of carbon, approximately 2.13%. Moreover, it is devoid of alloying additives, but contains impurities of silicon, manganese and magnesium.
Features of Alloy Steel
In addition to the carbon and iron content, additional elements are added to it to improve its properties.
Features of stainless steel
Stainless steel differs from carbon steel due to the content of the element chromium in its composition, due to the properties of which it is not susceptible to oxidation, and, therefore, to rust.
Features of tool steel
It also has a carbon composition (0.8 - 0.9%). Demonstrates hardness, strength, and is easy to process. Used in the manufacture of instruments, such as medical ones.
Wood's alloy
It is a material used in soldering parts for radio receivers, as well as in electroplating, when working in laboratory conditions with pesticides.
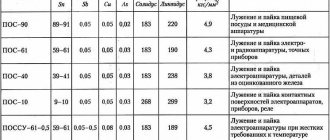
Soldering alloys
Another name for them is solders. Materials for solders are different. It all depends on what is included in the materials that need to be combined. For example, aluminum requires one alloy of solder, but copper is completely different.
Solder making
In order to improve the performance characteristics of the solder, a small amount of antimony is added to its composition. This type of solder is used for soldering various radio components, especially critical areas.
When choosing a solder, you should pay attention to the alloy containing silver . Its performance qualities can be called:
- The service life is significantly increased. Due to silver, the structure becomes more resistant to the oxidation process.
- By increasing the concentration of silver, it becomes possible to use solder in the manufacture of various parts of industrial equipment. However, silver significantly increases the cost of the alloy, as well as the manufactured product. This is why alloys with high concentrations of silver are used to make important parts.
Zinc is being added to the composition , but such alloys are less popular. This is due to the fairly high chemical activity of zinc. Due to interaction with the environment, such an alloy quickly deteriorates. Solder pastes are produced based on a zinc-containing mixture, which have a relatively short service life. The melting point in this case is 200 degrees Celsius.
Pure tin has also been used as a semiconductor solder for many years. The melting point of this element in its pure form is 240 degrees Celsius. They are used exclusively in industry, which is associated with high cost. In its pure form, due to a significant increase in temperature, the structure of tin is reconstructed, black spots appear on the surface, which indicate a significant deterioration in its basic qualities.