Aluminum is the most abundant metal in the earth's crust. It belongs to the group of light metals, has a low density and melting point. At the same time, ductility and electrical conductivity are at a high level, which ensures its widespread use. So, let's find out what the specific melting point of aluminum and its alloys is (for example, in comparison with iron and lead), thermal and electrical conductivity, density, other properties, as well as what are the features of the structure of aluminum alloys and their chemical composition.
History of discovery
In the 16th century, the famous Paracelsus took the first step towards aluminum mining. From alum, he isolated “alum earth,” which contained the oxide of a then unknown metal. In the 18th century, the German chemist Andreas Marggraff returned to this experiment. He named the aluminum oxide “alumina,” which means “astringent” in Latin. At that time, the metal was not popular because it was not found in its pure form. For many years, English, Danish and German scientists tried to isolate pure aluminum. In 1855, at the Paris World Exhibition, the metal aluminum created a sensation. Only luxury items and jewelry were made from it, since the metal was quite expensive. At the end of the 19th century, a more modern and cheaper method of producing aluminum appeared. In 1911, the first batch of duralumin, named after the city, was produced in Duren. In 1919, the first airplane was created from this material.
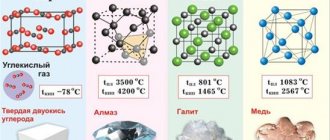
Specific electrical conductivity of some substances (table)
Specific conductivity is given at +20 °C:
| Substance | Sm/m | Substance | Sm/m | Substance | Sm/m | Substance | Sm/m | Substance | Sm/m |
| silver | 62 500 000 | molybdenum | 18 500 000 | tin | 8 330 000 | mercury | 1 040 000 | marble | 10−8 |
| copper | 59 500 000 | tungsten | 18 200 000 | cast steel | 7 690 000 | nichrome | 893 000 | glass | 10−11 |
| gold | 45 500 000 | zinc | 16 900 000 | lead | 4 810 000 | graphite | 125 000 | porcelain | 10−14 |
| aluminum | 38 000 000 | nickel | 11 500 000 | nickel silver | 3 030 000 | sea water | 3 | quartz glass | 10−16 |
| magnesium | 22 700 000 | pure iron | 10 000 000 | constantan | 2 000 000 | the ground is wet | 10−2 | amber | 10−18 |
| iridium | 21 100 000 | platinum | 9 350 000 | manganin | 2 330 000 | distilled water | 10−4 |
Physical properties
Aluminum metal is characterized by high electrical conductivity, thermal conductivity, resistance to corrosion and frost, and ductility. It lends itself well to stamping, forging, drawing, and rolling. Aluminum can be welded well with various types of welding. An important property is its low density of about 2.7 g/cm³. The melting point is about 660°C. The mechanical, physicochemical and technological properties of aluminum depend on the presence and amount of impurities that worsen the properties of the pure metal. The main natural impurities are silicon, iron, zinc, titanium and copper.
According to the degree of purification, aluminum is distinguished between high and technical purity. The practical difference is the difference in corrosion resistance to certain environments. The purer the metal, the more expensive it is. Technical aluminum is used for the production of alloys, rolled products and cable and wire products. High purity metal is used for special purposes. In terms of electrical conductivity, aluminum is second only to gold, silver and copper. And the combination of low density and high electrical conductivity allows it to compete with copper in the field of cable and wire products. Long-term annealing improves electrical conductivity, while cold hardening worsens it.
The thermal conductivity of aluminum increases with increasing purity of the metal. Impurities of manganese, magnesium and copper reduce this property. In terms of thermal conductivity, aluminum is inferior only to copper and silver. Due to this property, the metal is used in heat exchangers and cooling radiators. Aluminum has a high specific heat capacity and heat of fusion. These figures are significantly higher than those of most metals. The higher the purity of aluminum, the more it is able to reflect light from the surface. The metal is well polished and anodized.
Aluminum has a high affinity for oxygen and is covered in air with a thin, durable film of aluminum oxide. This film protects the metal from subsequent oxidation and provides its good anti-corrosion properties. Aluminum is resistant to atmospheric corrosion, sea and fresh water, and practically does not interact with organic acids, concentrated or diluted nitric acid.
Connection of copper and aluminum wires
Recently, electrical equipment of increasingly higher power has begun to be used in everyday life and industry. During Soviet times, wiring was made mainly of cheap aluminum. Unfortunately, its performance characteristics no longer meet the new requirements. Therefore, today in everyday life and in industry very often aluminum wires are replaced with copper ones. The main advantage of the latter, in addition to refractoriness, is that during the oxidation process their conductive properties do not decrease.
Nichrome
Often when modernizing electrical networks, aluminum and copper wires have to be connected. This cannot be done directly. Actually, the electrical conductivity of aluminum and copper does not differ too much. But only for these metals themselves. The oxidizing films of aluminum and copper have different properties. Because of this, the conductivity at the junction is significantly reduced. The oxidation film of aluminum has much greater resistance than that of copper. Therefore, the connection of these two types of conductors must be made exclusively through special adapters. These could be, for example, clamps containing a paste that protects metals from the appearance of oxide. This adapter option is usually used outdoors. Branch compressors are more often used indoors. Their design includes a special plate that eliminates direct contact between aluminum and copper. If such conductors are not available at home, instead of twisting the wires directly, it is recommended to use a washer and nut as an intermediate “bridge”.
Chemical properties
Aluminum is a fairly active amphoteric metal. Under normal conditions, a strong oxide film determines its durability. If the oxide film is destroyed, aluminum acts as an active reducing metal. In a finely crushed state and at high temperatures, the metal interacts with oxygen. When heated, reactions occur with sulfur, phosphorus, nitrogen, carbon, and iodine. Under normal conditions, the metal reacts with chlorine and bromine. There is no reaction with hydrogen. With metals, aluminum forms alloys containing intermetallic compounds - aluminides.
Provided that the oxide film is removed, vigorous interaction with water occurs. Reactions with dilute acids occur easily. Reactions with concentrated nitric and sulfuric acid occur when heated. Aluminum reacts easily with alkalis. Practical application in metallurgy has found the property of reducing metals from oxides and salts - aluminothermy reactions.
Strength of aluminum at low temperatures
Aluminum and its alloys, unlike steels, do not have a cold brittleness threshold. On the contrary, their strength increases with decreasing temperature. Prolonged exposure at low temperatures does not affect the level of strength properties of stable thermally strengthened aluminum alloys, either directly at these low temperatures or upon returning to room temperature.
Freshly quenched heat-hardening alloys can be maintained in this state for long periods of time if they are stored at low temperatures to delay the aging process. Aircraft rivets made of AlCuMgSi alloys (duralumin) are heated for hardening at 495 °C for 5 to 60 minutes, depending on the size and number of rivets, after which they are hardened in cold water. At room temperature, rivets remain plastic for two hours, at –5 °C this state persists for 45 hours, and at –15 °C – 150 hours!
The increase in the strength characteristics of aluminum alloys with decreasing temperature is almost not noticeable down to –15 °C, but begins to increase significantly below –100 °C. The figure shows the behavior of alloy 6061 in the temperature range from -250 °C to room temperature.
Receipt
Aluminum is in first place among metals and in third place among all elements in terms of abundance in the earth's crust. Approximately 8% of the mass of the earth's crust is this metal. Aluminum is found in the tissues of animals and plants as a trace element. In nature, it is found bound in the form of rocks and minerals. The rocky shell of the earth, which is at the base of the continents, is formed precisely by aluminosilicates and silicates.
Aluminosilicates are minerals formed as a result of volcanic processes under appropriate high temperature conditions. During the destruction of aluminosilicates of primary origin (feldspars), various secondary rocks with a higher aluminum content (alunites, kaolins, bauxites, nephelines) were formed. Aluminum is included in secondary rocks in the form of hydroxides or hydrosilicates. However, not every aluminum-containing rock can be a raw material for alumina, a product from which aluminum is produced using the electrolysis method.
Aluminum is most often obtained from bauxite. Deposits of this mineral are common in countries of the tropical and subtropical zone. In Russia, nepheline ores are also used, deposits of which are located in the Kemerovo region and on the Kola Peninsula. When extracting aluminum from nephelines, potash, soda ash, cement and fertilizers are also produced along the way.
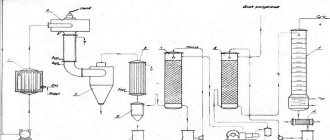
Bauxite contains 40-60% alumina. It also contains iron oxide, titanium dioxide, and silica. The Bayer process is used to isolate pure alumina. In an autoclave, the ore is heated with caustic soda, cooled, and the “red mud” (solid sediment) is separated from the liquid. Afterwards, aluminum hydroxide is precipitated from the resulting solution and calcined to obtain pure alumina. Alumina must meet high standards for purity and particle size.
Alumina (aluminum oxide) is extracted from the mined and enriched ore. The alumina is then converted into aluminum using electrolysis. The final stage is recovery by the Hall-Heroux process. The process is as follows: during the electrolysis of an alumina solution in molten cryolite, aluminum is released. The cathode is the bottom of the electrolysis bath, and the anode is carbon bars located in cryolite. Molten aluminum is deposited under a solution of cryolite with 3-5% alumina. The process temperature rises to 950°C, which is much higher than the melting point of aluminum itself (660°C). Deep purification of aluminum is carried out by zone melting or distillation through subfluoride.
Electrical conductivity and current carriers
The electrical conductivity of all substances is associated with the presence of current carriers (charge carriers) in them - mobile charged particles (electrons, ions) or quasiparticles (for example, holes in a semiconductor) capable of moving in a given substance over a long distance; in a simplified way, we can say what is meant that such a particle or quasiparticle should be able to travel an arbitrarily large, at least macroscopic, distance in a given substance, although in some particular cases carriers can change, being born and destroyed (generally speaking, sometimes, perhaps, through a very short distance), and carry current, replacing each other.
Since the current density is determined for one type of carrier by the formula:
j→=qnv→cp.,{\displaystyle {\vec {j}}=qn{\vec {v}}_{cp.},} where q{\displaystyle q} is the charge of one carrier, n{\displaystyle n} is the concentration of carriers, v→cp.{\displaystyle {\vec {v}}_{cp.}} is the average speed of their movement,
or j→=∑iqiniv→icp.{\displaystyle {\vec {j}}=\sum _{i}q_{i}n_{i}{\vec {v}}_{icp.}} for more than one type of carrier, numbered by the index i,{\displaystyle i,} taking a value from 1 to the number of types of carriers, each of which can have its own charge (possibly different in magnitude and sign), its own concentration, its own average speed of movement (summation in this formula is implied for all available types of carriers), then, given that the (steady-state) average speed of each type of particle when moving in a specific substance (medium) is proportional to the applied electric field (in the case when the movement is caused precisely by this field, which is what we are here we are considering):
v→cp.=μE→,{\displaystyle {\vec {v}}_{cp.}=\mu {\vec {E}},} where μ{\displaystyle \mu } is a proportionality coefficient called mobility and depending on the type of current carrier in a given specific environment.
It follows that the expression for electrical conductivity is valid:
σ=qnμ,{\displaystyle \sigma =qn\mu ,}
or:
σ=∑iqiniμi{\displaystyle \sigma =\sum _{i}q_{i}n_{i}\mu _{i}} - for more than one type of media.
Application
Aluminum is used in metallurgy as a base for alloys (duralumin, silumin) and an alloying element (alloys based on copper, iron, magnesium, nickel). Aluminum alloys are used in everyday life, in architecture and construction, in shipbuilding and automotive industry, as well as in space and aviation technology. Aluminum is used in the production of explosives. Anodized aluminum (coated with colored films of aluminum oxide) is used to make jewelry. Metal is also used in electrical engineering.
Structural steels.
They are classified according to the characteristics and chemical composition of the alloys. If they are high quality and ordinary. Both are carbon steels, although the carbon content in them is insignificant.
The purpose of ordinary structural alloys is the manufacture of industrial products that must be subjected to serious mechanical loads: nails, bolts, angles, channels, beams, etc. High-quality structural steels are suitable for the manufacture of parts used in mechanical engineering. Of course, their withstand loads are much lower, such grades have become much softer, they are used for the manufacture of parts by cold stamping. In addition, there are especially high-quality brands, they are called cryogenic. They retain their strength characteristics at extremely low temperatures. They are used to make containers for transporting and storing liquefied gases, and are also used in the construction of facilities in permafrost conditions.