Isotopes
Main article: Isotopes of tin
Natural tin consists of ten stable nuclides with mass numbers 112 (in a mixture of 0.96% by mass), 114 (0.66%), 115 (0.35%), 116 (14.30%) , 117 (7.61%), 118 (24.03%), 119 (8.58%), 120 (32.85%), 122 (4.72%), and 124 (5.94%). For some of them, double beta decay is energetically possible, but it has not yet been observed experimentally (2018) because the predicted half-life is very long (more than 1020 years).
Tin has the largest number of stable isotopes of all elements, which is due to the fact that 50 (the number of protons in tin nuclei) is the magic number - it constitutes a filled proton shell in the nucleus and thereby increases the binding energy and stability of the nucleus. Two doubly magic isotopes of tin are known, both of them are radioactive because they are far from the beta stability band: neutron-deficient 100Sn ( Z
=
N
= 50) and neutron-rich 132Sn (
Z
= 50,
N
= 82).
Tin isotopes 117Sn and 119Sn are Mössbauer isotopes and are used in gamma resonance spectroscopy.
Chemical properties
Metal tin
At room temperature, tin, like its group neighbor germanium, is resistant to air or water. This inertness is explained by the formation of a surface film of oxides. Noticeable oxidation of tin in air begins at temperatures above 150 °C:
Sn + O2 → SnO2
When heated, tin reacts with most non-metals. In this case, compounds are formed in the oxidation state +4, which is more characteristic of tin than +2. For example:
Sn + 2Cl2 → SnCl4
Dissolves in dilute acids (HCl, H2SO4):
Sn + 2HCl → SnCl2 + H2↑
Tin reacts with concentrated hydrochloric acid. In this case, white tin (α-Sn) forms a solution of tin (II) chloride, and gray (β-Sn) tin (IV) chloride:
Sn + 3HCl → H[SnCl3 ] + H2↑ Sn + 4HCl → H2[SnCl6] + H2↑
The composition of the reaction product of tin with nitric acid depends on the concentration of the acid. In concentrated nitric acid (60%) stannous acid β-SnO2 n
H2O (sometimes its formula is written as H2SnO3).
In this case, tin behaves like a non-metal: Sn + 4HNO3 → SnO2 ⋅ H2O + 4NO2 + H2O
When interacting with dilute nitric acid (3-5%), tin (II) nitrate is formed:
4Sn + 10HNO3 → 4Sn(NO3)2 + NH4NO3 + 3H2O
It is oxidized by alkali solutions to hydroxostannate (II), which is prone to disproportionation in hot solutions:
Sn + NaOH + 3H2O → Na[Sn(OH)3] + H2↑ 2Na[Sn(OH)3] → Sn + Na2[Sn(OH)6] Sn + 2NaOH + 4H2O → Na2[Sn(OH)6] + 2H2↑
Tin(II)
Less stable oxidation state than (IV). The substances have high reducing activity and easily disproportionate:
2SnO →to SnO2 + Sn
In air, compounds are quickly oxidized by oxygen, both in solid form and in solutions:
2SnO + O2 → 2SnO2 2Sn2+ + O2 + 4H+ → 2Sn4+ + 2H2O
A strong reducing agent is the “tin salt” SnCl2 ⋅ 2H2O
The oxide can be obtained by the action of ammonia on a hot solution of tin chloride in a CO2 atmosphere:
SnCl2 + 2NH3 + H2O → SnO + 2NH4Cl
Also, when gently heating tin(II) hydroxide Sn(OH)2 in a vacuum or gently heating certain salts:
Sn(OH)2 → SnO + H2O SnC2O4 → SnO + CO↑ + CO2↑
In solutions of tin salts, strong hydrolysis occurs:
[Sn(H2O)3]2+ + H2O → [Sn(H2O)2OH]+ + H3O+
When a solution of Sn(II) salt is exposed to sulfide solutions, a precipitate of tin (II) sulfide precipitates:
Sn2+ + S2− → SnS↓
This sulfide can be easily oxidized to a sulfide complex with a solution of sodium polysulfide, which upon acidification turns into a precipitate of tin (IV) sulfide:
SnS + Na2S2 → Na2SnS3 Na2SnS3 + 2HCl → SnS2 + 2NaCl + H2S↑
Tin(IV)
Tin(IV) oxide (SnO2) is formed by direct oxidation with oxygen. When fused with alkalis, it forms stannates; when treated with water, it forms hydroxostannates:
SnO2 + 2NaOH →to Na2SnO3 + H2O
When hydrolyzing solutions of tin (IV) salts, a white precipitate is formed - the so-called α-tin acid:
SnCl4 + 4NH3 + 6H2O → H2[Sn(OH)6] + 4NH4Cl H2[Sn(OH)6] → SnO2 ⋅ nH2O + 3H2O
Freshly obtained α-stannous acid dissolves in acids and alkalis:
SnO2 ⋅ nH2O + 2KOH → K2[Sn(OH)6] SnO2 ⋅ nH2O + 4HNO3 → Sn(NO3)4 + (n+2)H2O
During storage, α-stannous acid ages, loses water and turns into β-stannous acid, which is more chemically inert. This change in properties is associated with a decrease in the number of active HO-Sn groups when standing and their replacement with more inert bridging -Sn-O-Sn- bonds.
Tin hydride - stannane SnH4 - can be obtained by the reaction:
SnCl4 + Li[AlH4] → SnH4↑ + LiCl + AlCl3
This hydride is very unstable and slowly decomposes even at a temperature of 0 °C.
Tetravalent tin forms a large class of organotin compounds used in organic synthesis, as pesticides, etc.
Types of Tin
Types of tin for soldering:
- POS-18. Contains several main components - tin (18%), lead (about 81), antimony (2.5%). Used for tinning metals. Suitable for creating seams with low standards. Melting point - 270°C.
- POS-30. Contains tin (28%), lead (about 70%), antimony (2%). Used for soldering copper, steel, brass. Melting point - 270°C.
- POS-50. Contains tin (50%), lead (about 50%), antimony (0.8%). It is used for soldering radio components, obtaining high-quality seams. Melting point - 230°C.
- POS-90. Contains tin (90%), lead (9–10%), antimony (0.15%).
Certain types of tin solders are POS-40, POS-60. Used for soldering radio components.
Being in nature
Tin is a rare trace element; tin ranks 47th in terms of abundance in the earth’s crust. The Clark content of tin in the earth's crust ranges, according to various sources, from 2⋅10−4 to 8⋅10−3% by mass. The main mineral of tin is cassiterite (tin stone) SnO2, containing up to 78.8% tin. Much less common in nature is stannin (tin pyrite) - Cu2FeSnS4 (27.5% Sn).
Place of Birth
The world's tin deposits are located mainly in China and Southeast Asia - Indonesia, Malaysia and Thailand. There are also large deposits in South America (Bolivia, Peru, Brazil) and Australia.
In Russia, tin ore reserves are located in the Khabarovsk Territory (Solnechny district - Festivalnoye and Sobolinoye deposits; Verkhnebureinsky district - Pravourmiyskoye deposit), in the Chukotka Autonomous Okrug (Pyrkakai stockworks; Valkumey mine/village, Iultin - deposit development was closed in the early 1990s) , in the Primorsky Territory (Kavalerovsky district), in Yakutia (Deputatskoye deposit) and other areas.
Prevalence in nature
The prevalence in nature is shown in the following table:
| Geol. an object | Kamen. meteorites | Dunits and others. | Basalts, etc. | Diorites, etc. | Granitoids | Glina, etc. | Ocean water | Live matter (% of live weight) | The soil | Plant ash |
| Contents, weight. % | 001⋅10−4 | 05⋅10−5 | 01,5⋅10−4 | 0000− | 0003⋅10−4 | 1⋅10−3 | 07⋅10−7 | 00005⋅10−5 | 1⋅10−3 | 005⋅10−4 |
In unpolluted surface waters, tin is found in submicrogram concentrations. In groundwater its concentration reaches several micrograms per liter, increasing in the area of tin ore deposits, it enters the water due to the destruction primarily of sulfide minerals, which are unstable in the oxidation zone. PDKSn = 2 mg/dm³.
Cassiterite crystals - tin ore
Tin is an amphoteric element, that is, an element capable of exhibiting acidic and basic properties. This property of tin also determines the characteristics of its distribution in nature. Due to this duality, tin exhibits lithophilic, chalcophilic and siderophilic properties. Tin in its properties is close to quartz, as a result of which the close connection of tin in the form of oxide (cassiterite) with acidic granitoids (lithophilicity), often enriched in tin, is known, up to the formation of independent quartz-cassiterite veins. The alkaline behavior of tin is determined by the formation of quite a variety of sulfide compounds (chalcophilicity), up to the formation of native tin and various intermetallic compounds known in ultrabasic rocks (siderophilicity).
Forms of location
The main form of occurrence of tin in rocks and minerals is scattered (or endocript). However, tin also forms mineral forms, and in this form it is often found not only as an accessory in acidic igneous rocks, but also forms industrial concentrations mainly in oxide (cassiterite SnO2) and sulfide (stannine) forms.
Solid phase. Minerals
Cassiterite crystals
In general, the following forms of tin occurrence in nature can be distinguished:
- Scattered form
; the specific form of tin in this form is unknown. Here we can talk about an isomorphically dispersed form of tin occurrence due to the presence of isomorphism with a number of elements (Ta, Nb, W - with the formation of typically oxygen compounds; V, Cr, Ti, Mn, Sc - with the formation of oxygen and sulfide compounds). If tin concentrations do not exceed certain critical values, then it can isomorphically replace the named elements. The mechanisms of isomorphism are different. - Mineral Form
: Tin is found in concentrating minerals. As a rule, these are minerals in which Fe+2 iron is present: biotites, garnets, pyroxenes, magnetites, tourmalines, and so on. This connection is due to isomorphism, for example, according to the scheme Sn+4 + Fe+2 → 2Fe+3. In tin-bearing skarns, high concentrations of tin are found in garnets (up to 5.8 wt.%) (especially in andradites), epidotes (up to 2.84 wt.%), and so on.
In sulfide deposits, tin is included as an isomorphic element in sphalerites (Silinskoye deposit, Russia, Primorye), chalcopyrites (Dubrovskoye deposit, Russia, Primorye), and pyrites. High concentrations of tin were detected in pyrrhotite from greisen from the Smirnovskoe deposit (Russia, Primorye). It is believed that due to limited isomorphism, solid solutions decompose with micro-precipitates of Cu2+1Fe+2SnS4 or tillite PbSnS2 and other minerals.
Actually mineral forms
Native elements, alloys and intermetallic compounds
Although the concentrations of these minerals in rocks are very low, they are distributed in a wide range of genetic formations. Among the native forms, along with Sn, Fe, Al, Cu, Ti, Cd, and so on were identified, not counting the already known native platinoids, gold and silver. These same elements also form various alloys with each other: (Cu + Sn + Sb), (Pb + Sn + Sb) and others, as well as solid solutions. Among the intermetallic compounds, stistaite SnSb, atakite (Pd,Pt)3Sn, shtumyrlite Pt(Sn,Bi), zvyagintsevite (Pd,Pt)3(Pb,Sn), taymyrite (Pd,Cu,Pt)3Sn and others were identified.
The following forms of occurrence of tin and other elements are found in various geological formations:
- A group of intrusive and effusive igneous rocks: traps, picrites of the Siberian Platform, hyperbasites and gabbroids of Kamchatka, kimberlites of Yakutia, lamproites of Aldan, and so on; granitoids of Primorye, Far East, Tien Shan.
- A group of metasomatically and hydrothermally altered rocks: copper-nickel ores of the Siberian platform, gold deposits of the Urals, the Caucasus, Uzbekistan, and so on.
- Group of modern ore formation: pelagic sediments of the Pacific Ocean, products of the Great Fissure Tolbachik eruption, Uzon hydrothermal system in Kamchatka and others.
- A group of sedimentary rocks of various origins.
Tin oxide compounds
The most famous form is the main mineral of tin - cassiterite SnO2, which is a compound of tin with oxygen. According to nuclear gamma resonance spectroscopy, the mineral contains Sn+4.
Cassiterite
Main article: Cassiterite
Cassiterite
(from the Greek kassiteros - tin) - the main ore mineral for the production of tin, chemical formula SnO2. Theoretically contains 78.62% Sn. It forms separate secretions, grains, continuous massive aggregates, in which the mineral grains reach a size of 3-4 mm or even more. In its pure form, colorless crystals; impurities give the mineral a variety of colors.
- Density 6040-7120 kg/m³ (lowest for light-colored cassiterites).
- Mohs hardness 6.5.
- The shine is matte, the edges are diamond-like.
- Cleavage is imperfect.
- The fracture is conchoidal.
The main forms of cassiterite isolation:
- microinclusions in other minerals;
- accessory mineral deposits in rocks and ores;
- solid or disseminated ores: needle-shaped radial aggregates (Primorye deposits), colomorphic and cryptocrystalline segregations and accumulations (Primorye deposits); The crystalline form is the main form of cassiterite isolation.
In Russia, cassiterite deposits are found in the Northeast, Primorye, Yakutia, and Transbaikalia; abroad - in Malaysia, Thailand, Indonesia, China, Bolivia, Nigeria and other countries.
Hydroxide compounds
Tin hydroxide compounds occupy a secondary place
, which can be considered as salts of polytin acids. These include the mineral succulite Ta2Sn2+2O; solid solution of tin in magnetite of the type Fe2SnO4 or Fe3SnO3 (Brettstein Yu. S., 1974; Voronina L. B., 1979); “varlamovit” is a product of stannine oxidation; it is believed to be a mixture of amorphous and semi-amorphous Sn compounds, metatinic acid, a polycondensed phase and a hydrocassiterite phase. Hydrated oxidation products are also known - hydromartite 3SnO·H2O; mushistonite (Cu,Zn,Fe)Sn(OH)6; copper hydrostannate CuSn(OH)6 and others.
Silicates
A large group of tin silicates
, represented by malayaite CaSn[SiO5]; pabstite Ba(Sn, Ti)Si3O9, stocasite Ca2Sn2Si6O18·4H2O, etc. Malayaite even forms industrial accumulations.
Spinelids
Spinels are also known among other oxide compounds.
, for example, the mineral nigerite Sn2Fe4Al16O32 (Peterson EU, 1986).
Tin sulfide compounds
Includes various tin and sulfur compounds. This is the second most industrially important group of mineral forms of tin. The most important of these is stannine, the second most important mineral. In addition, frankeite Pb5Sn3Sb2S14, herzenbergite SnS, berndtite SnS2, tillite PbSnS2 and kesterite Cu2ZnSnS4 are noted. More complex sulfide compounds of tin with lead, silver, and copper, which are mainly of mineralogical significance, have also been identified. The close connection of tin with copper determines the frequent presence of chalcopyrite CuFeS2 in tin ore deposits with the formation of the cassiterite-chalcopyrite paragenesis.
Stannin
Main article: Stannin
Stannine (from Latin stannum - tin), tin pyrite, a mineral from the class of sulfides with the general formula of the form Cu2FeSnS4. It follows from the chalcopyrite formula by replacing one Fe atom with Sn. Contains 29.58% Cu, 12.99% Fe, 27.5% Sn and 29.8 S, as well as impurities of Zn, Sb, Cd, Pb and Ag. A widespread mineral in tin ore deposits in Russia. In a number of deposits in Russia (Primorye, Yakutia) and Central Asia (Tajikistan), it is an essential element of sulfide minerals and often, together with varlamovite, makes up 10-40% of total tin. Often forms impregnations in ZnS sphalerite and chalcopyrite. In many cases, stannine decomposition phenomena with the release of cassiterite are observed.
Colloidal form
Colloidal and tin-silicon compounds play a significant role in the geochemistry of tin, although it has not been studied in detail. A significant place in the geology of the element is played by colomorphic compounds and the products of its crystalline transformations into cryptocrystalline varieties. Colomorphic cassiterite is considered as a form of expression of viscous gel-like solutions.
Independent studies have revealed an abnormally high solubility of SnO2 in chlorine-silicon solutions. Maximum solubility is achieved at a ratio of SnO2\SiO2 = 1.5.
Analysis of the properties of the Sn(OH)4 compound and their proximity to the Si(OH)4 compound reveals its ability to polymerize with the eventual formation of H2Sn k
O2
k
+1, Sn
k
O2
k
−1(OH)2. In both cases, it is possible to replace the (OH) group with the F and Cl anions.
Thus, the polymerization of Sn(OH)4 molecules and their combination with Si(OH)4 molecules leads to the formation of a gel (colloid) and the appearance of H m
Sn2
n
Si
n
O
p
, with
m
≤ 8, or H
s
[SiO2
n
(SnO
m
)
d
] (Nekrasov I. Ya. et al., 1973).
Available evidence suggests that the colloidal form is a natural intermediate in the precipitation of tin from hydrothermal solutions.
Forms of tin in the liquid phase
The least studied part of the geochemistry of tin, although cassiterites in the form of prisoner minerals have been established in gas-liquid inclusions (Kokorin A. M. et al., 1975). There are no works on the analysis of specific tin-containing natural solutions. Basically, all information is based on the results of experimental studies, which speak only about the probable forms of tin in solutions. A significant role in the development of the methodology for these studies belongs to Academician V.L. Barsukov.
The entire set of experimentally established forms of tin in solutions is divided into groups:
- Ionic compounds
.
These compounds and their structure are described in terms of classical valence and stereochemical concepts. Subgroups are distinguished: Simple ions
Sn+2 and Sn+4 are mainly found in magmatic melts, as well as in hydrothermal solutions with low pH values. However, in existing hydrothermal systems, reflected by the composition of gas-liquid inclusions, such conditions have not been established. - Halides
- SnF2, SnF40, SnCl40. The role of chlorine in the transport and deposition of tin and associated metals is believed to be more significant than that of fluorine. - Hydroxyl compounds
.
Under alkaline conditions, the starting compounds are H2SnO2, H2SnO4, H2SnO3. These forms are often established based on known mineral forms. Some of these forms are of both artificial (CaSnO3, Ca2SnO4) and natural (FeSnO2, Fe2SnO4) origin. In acidic environments, these compounds behave as weak bases such as Sn(OH)2, Sn(OH)4. It is believed that one of the forms of manifestation of such compounds is varlamovit. According to experimental data, Sn(OH)4 is deposited only at T
< 280 °C in slightly acidic or neutral conditions at pH = 7-9.
The compounds Sn(OH)4 and Sn(OH)3+ are stable at pH = 7-9, while Sn(OH)2+2 and Sn(OH)+2 are stable at pH < 7. Quite often (OH)− groups 1 are replaced by F and Cl, creating halogen-substituted modifications of hydrotin compounds. In general, these forms are represented by the compounds Sn(OH)4- k
F
k
or Sn(OH)4−
k
F
kn
Cl
n
.
In general, the Sn(OH)3F compound is stable at T
= +25...+50 °C, and Sn(OH)2F2 - at
T
= 200 °C. - Sulfide compounds
. According to experimental data, the solution contains SnS4−4 or SnS3−2 compounds at pH > 9; SnS2O−2 (pH = 8–9) and Sn(SH)4 (pH = 6). There is mention of the existence of a compound of the Na2SnS3 type, which is unstable in an acidic environment.
tin compounds have been studied during the dissolution of cassiterite in fluorinated media.
These compounds are highly soluble. Compounds obtained in chloride solutions have the same properties. The main forms of complex compounds known from experiments include Na2[Sn(OH)6], Na2[SnF6], Na2[Sn(OH)2F4], etc. Experiments have shown that the complex Sn(OH)4F2−2 will prevail at T
= 200 °C.
. Their existence is evidenced by the presence of colomorphic cassiterite segregations in many deposits.
Industrial types of tin deposits
The geochemical features of tin described above are indirectly reflected in the formational classification of tin ore deposits proposed by E. A. Radkevich with subsequent additions.
A.
Formation of tin-bearing granites
.
Cassiterite is installed in the accessory part of granites. B. Formation of rare metal granites
.
These are granites of the lithionite-amazonite-albite type (apogranites according to A. A. Beus). Cassiterite in the accessory part together with columbite-tatnatlite, microlite and others. B. Formation of tin-bearing pegmatites
.
Tin mineralization is characteristic of Be-Li-, Be-Ta-, F-Li- types. D. Feldspar-quartz-cassiterite formation
.
Iv is highlighted. F. Grigoriev. These are quartz-feldspar veins with cassiterite and other minerals. D. Quartz-cassiterite formation
.
Distributed in northeast Russia. These are vein zones, greisens with quartz, muscovite, wolframite, cassiterite and others. E. Cassiterite-silicate-sulfide formation
with tourmaline and chlorite types.
One of the main productive formations of Primorye Russia. G. Cassiterite-sulfide formation
.
Also the main tin-producing formation. It distinguishes the main types: 1) stockwork tin-tungsten mineralization; 2) ore bodies of quar-cassiterite-arsenopyrite type; 3) productive quartz veins of the sulfide-cassiterite-chlorite type. H. Tin-skarn formation
.
I. Woody tin formation
(rhyolite formation).
K. Formation of basic and ultrabasic rocks
(according to I. Ya. Nekrasov).
L. Formation of alkaline rocks of Ukraine
(according to V.S. Metallidi, 1988).
How to distinguish tin from solder?
First of all, to distinguish pure tin from solder, you need to know that it crunches without impurities. If you take a tin rod and try to break a piece from it, you will hear a crunch.
Also, when determining tin, it is recommended to look at the following:
- Weight. Solder containing lead will always be heavier than tin. Therefore, if you pick up tin and solder or put it on a scale, the difference in weight will be more than noticeable;
- Appearance. In appearance, pure tin has a silvery and slightly white tint, while lead is always darkish. This happens due to the fact that lead actively oxidizes in air, so it darkens greatly;
- Pure tin crunches when deformed. The difference between lead solder is that its structure is soft. Solder can be easily cut, bent, broken, and there will not be such a crunch as pure tin.
Most often, people need to know whether the tin in front of them is pure or not when making certain things. No one would want to carry items that contain lead because everyone knows that lead is harmful and can cause serious health problems over time.
Production
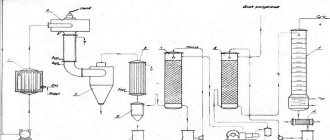
During the production process, the ore-bearing rock (cassiterite) is crushed to particle sizes of an average of ~10 mm in industrial mills, after which cassiterite, due to its relatively high density and mass, is separated from the waste rock by vibration-gravity method on dressing tables. In addition, the flotation method of ore enrichment/purification is used. In this way, it is possible to increase the tin content in the ore to 40-70%. Next, the concentrate is roasted in oxygen to remove impurities of sulfur and arsenic. The resulting tin ore concentrate is smelted in furnaces. During the smelting process, it is restored to a free state through the use of charcoal in the reduction, the layers of which are laid alternately with layers of ore, or aluminum (zinc) in electric furnaces: SnO2 + C = Sn + CO2. Particularly pure tin of semiconductor purity is prepared by electrochemical refining or the zone melting method.
Tin brands
The following grades of tin are known:
- O1, O1pch. This designation indicates that the alloy contains 99.9% Sn. It is made in the form of wire, rods, ingots.
- VHF-000. High purity alloy. The Sn content in the composition is 99.99%. It is made in the form of rods and ingots.
- O2. The Sn content in the composition is 99.565%. Produced in the form of rods, wire, pigs.
- O3. The alloy contains 98.49% Sn. Made in ingots.
- O4. The dirtiest connection. Contains a large number of foreign impurities. Their approximate amount is 3.5% of the total mass.
Marking is indicated on finished products using a stamp.
Application
- Tin is used primarily as a safe, non-toxic, corrosion-resistant coating in its pure form or in alloys with other metals. The main industrial uses of tin are in tinplate (tinned iron) for food containers, in solders for electronics, in household piping, in bearing alloys, and in coatings of tin and its alloys. The most important alloy of tin is bronze (with copper). Another well-known alloy, pewter, is used to make tableware. About 33% of all mined tin is consumed for these purposes. Up to 60% of tin produced is used in the form of alloys with copper, copper and zinc, copper and antimony (bearing alloy, or babbitt), with zinc (packaging foil) and in the form of tin-lead and tin-zinc solders. Recently, there has been a revival of interest in the use of metal, since it is the most “ecologically friendly” among heavy non-ferrous metals. Used to create superconducting wires based on the Nb3Sn intermetallic compound.
- Tin disulfide SnS2 is used in paints that imitate gold leaf (“potal”).
- Artificial radioactive nuclear isomers of tin 117mSn and 119mSn are sources of gamma radiation, are Mössbauer isotopes and are used in gamma resonance spectroscopy.
- Intermetallic compounds of tin and zirconium have high melting points (up to 2000 °C) and resistance to oxidation when heated in air and have a number of applications.
- Tin is the most important alloying component in the production of structural titanium alloys.
- Tin dioxide is a very effective abrasive material used to “finish” the surface of optical glass.
- A mixture of tin salts - the "yellow composition" - was previously used as a dye for wool.
- Tin is also used in chemical current sources as an anode material, for example: manganese-tin element, mercury-tin oxide element. The use of tin in a lead-tin battery is promising; for example, at the same voltage, compared to a lead battery, a lead-tin battery has 2.5 times greater capacity and 5 times greater energy density per unit volume, its internal resistance is much lower.
- Isolated two-dimensional layers of tin (stanene), created by analogy with graphene, are studied.
Obtaining tin from ore and deposits
The process of obtaining the alloy depends on the form in which it was found. Tin in the form of ore does not differ significantly from the production of other non-ferrous metals. The process consists of three stages:
- Extraction, processing of consumable raw materials (ore).
- Reduction smelting to obtain rough metal.
- Refining prepared raw materials using acceptable methods.
The development of placer deposits is carried out using industrial sand pumps.
Physiological action
Almost nothing is known about the role of tin in living organisms. The daily intake of tin from food is 0.2-3.5 mg, with regular consumption of canned food - up to 38 mg. The human body contains approximately (1-2)·10−4% tin, the highest concentration is observed in the intestines.
Tin metal is non-toxic, which allows it to be used in the food industry. Tin poses a danger to humans in the form of vapors, various aerosol particles and dust. When exposed to tin vapors or dust, stannosis can develop - lung damage. Stannan (tin hydrogen) is a powerful poison. Some organotin compounds are also very toxic. The temporary permissible concentration of tin compounds in atmospheric air is 0.05 mg/m3, the maximum permissible concentration of tin in food products is 200 mg/kg, in dairy products and juices - 100 mg/kg. The toxic dose of tin for humans is 2 g; intoxication of the body begins when the body contains 250 mg/kg.
Harmful impurities contained in tin under normal conditions of storage and use, including in the melt at temperatures up to 600 °C, are not released into the air in volumes exceeding the maximum permissible concentration (in particular, determined according to GOST 12.1.005-76. Long-term (for 15-20 years) exposure to tin dust has a fibrogenic effect on the lungs and can cause pneumoconiosis in workers.
general description
People began mining metal ore back in the 4th century BC. Ancient Greek and Roman objects were made from tin bronze, which was common in those days. The alloys also contained an admixture of lead and copper, and they learned to obtain pure metal only in the 7th century.
The rare element ranks 46th in abundance in the earth's crust. It occurs in the form of cassiterite, the mass of which contains up to 78% tin. Less common is tin pyrite with an admixture of copper and iron. Tin belongs to the group of amphoteric substances. The element is capable of exhibiting basic and acidic characteristics.
The metal forms individual quartz-cassiterite veins due to the close connection of oxygen tin compounds with granite anhydrites. Alkaline properties are manifested in the formation of various sulfide compounds, up to the occurrence of intermetallic mergers and native tin alloy in basic rocks.
White and gray tin
There are several allotropic modifications of tin. Under normal conditions, there is white tin, which is stable at temperatures above +13.3˚C. This is a soft metal that forms crystals with unit cells, where two identical vectors and a third different one are located strictly perpendicular to each other. A characteristic crunch is heard when the rod is bent. Sound occurs when crystals rub together.
Cooling the substance leads to the formation of gray tin. In this case, cubic crystals with a diamond structure appear. Ionizing radiation also promotes the transition from one modification to another and crystallization of the carbonate type.
Transformation of the structure leads to the following changes:
- the specific volume increases;
- the density of tin decreases;
- the metal becomes powdery.
The electrical properties of the two variations differ due to differences in structural lattices and valency. White tin belongs to the group of metals, and gray tin receives the characteristics of a covalent crystal with a diamond structure. The contact of two modifications leads to an acceleration of the electronic phase transition, as new forms of crystals are born. This phenomenon is called the tin plague. A stabilizer (eg bismuth) is used to prevent this process. The catalyst ammonium hexachlorostannate, on the contrary, accelerates the transition.
Isotopes of the element
Tin in nature contains 10 unchanged nuclides with a certain total number of neutrons, protons and electrons in the molecule. The atomic charge is also constant and corresponds to the atomic number of the element in the periodic table in chemistry.
Mass numbers of nuclides with changes in mass content:
- 112 - 0.96% in the mixture.
- 114 — 0,66%.
- 115 — 0,35%.
- 116 — 14,3%.
- 117 — 7,61%.
- 118 — 24,03%.
- 119 — 8,58%.
- 120 — 32,85%.
- 122 — 4,72%.
- 124 — 5,94%.
Some elements can undergo energetic double decay, but such a phenomenon has not been observed until now due to the theoretical decay time of 1020 years. Tin produces the largest number of stable isotopes. They fill the proton capsule and increase the stability of the nucleus.
Actually mineral forms
Native elements, alloys and intermetallic compounds
Although the concentrations of these minerals in rocks are very low, they are distributed in a wide range of genetic formations. Among the native forms, along with Sn, Fe, Al, Cu, Ti, Cd, etc. were identified, not counting the already known native platinoids, gold and silver. These same elements also form various alloys with each other: (Cu + Sn + Sb), (Pb + Sn + Sb), etc., as well as solid solutions. Among the intermetallic compounds, stistaite SnSb, atakite (Pd,Pt)3Sn, shtumyrlite Pt(Sn,Bi), zvyagintsevite (Pd,Pt)3(Pb,Sn), taymyrite (Pd,Cu,Pt)3Sn and others were identified.
The following forms of occurrence of tin and other elements are found in various geological formations:
- A group of intrusive and effusive igneous rocks: traps, picrites of the Siberian platform, hyperbasites and gabbroids of Kamchatka, kimberlites of Yakutia, lamproites of Aldan, etc.; granitoids of Primorye, Far East, Tien Shan.
- A group of metasomatically and hydrothermally altered rocks: copper-nickel ores of the Siberian platform, gold deposits of the Urals, the Caucasus, Uzbekistan, etc.
- Group of modern ore formation: pelagic sediments of the Pacific Ocean, products of the Great Fissure Tolbachik eruption, Uzon hydrothermal system in Kamchatka, etc.
- A group of sedimentary rocks of various origins.
origin of name
Latin name stannum
, related to the Sanskrit word meaning "steadfast, durable", originally referred to an alloy of lead and silver, and later to another alloy imitating it, containing about 67% tin; by the 4th century, this word began to be used to refer to tin itself.
The word tin
- Common Slavic, which has correspondences in the Baltic languages (cf. Lit.
alavas
,
alvas
- “tin”, Prussian
alwis
- “lead”).
It is a suffix from the root ol-
(cf. Old High German
elo
- “yellow”, Latin
albus
- “white”, etc.), so the metal is named by color.
Production
The world's cassiterite deposits are developed in Southeast Asia, mainly in China, Indonesia, Malaysia and Thailand. Other important deposits of cassiterite are located in South America (Bolivia, Peru, Brazil) and Australia. In Russia, tin ore reserves are located in the Chukotka Autonomous Okrug (Valkumey mine/village, development of the deposit was closed in the early 90s), in the Primorsky Territory (Kavalerovsky district), in the Khabarovsk Territory (Solnechny district, Verkhnebureinsky district (Pravourmiyskoye deposit)), in Yakutia (Deputatskoye deposit) and other areas.
Advantages and disadvantages of tin
The benefits of trapping include:
- Plastic. Complex products for interior decoration are made from tin.
- Inertia. The metal is used in the food industry for the manufacture of dishes and containers for storing food.
- Low melting point. Tin is used to apply a protective coating to metal parts.
Flaws:
- Low strength index. The alloy is not suitable for the manufacture of parts that will be subject to heavy loads.
- Rarity. Because of this, the price of the material increases.
Cassiterite article: Cassiterite
Cassiterite
(from the Greek kassiteros - tin) - the main ore mineral for the production of tin. Theoretically contains 78.62% Sn. It forms separate secretions, grains, continuous massive aggregates, in which the grains of the mineral reach a size of 3 - 4 mm and even more.
- Density 6040-7120 kg/m³ (lowest for light-colored cassiterites).
- Hardness 6½.
- The shine is matte, the edges are diamond-like.
- Cleavage is imperfect.
- The fracture is conchoidal.
The main forms of cassiterite isolation:
- microinclusions in other minerals;
- accessory mineral deposits in rocks and ores;
- solid or disseminated ores: needle-shaped radial aggregates (Primorye), colomorphic and cryptocrystalline segregations and accumulations (Primorye); The crystalline form is the main form of cassiterite isolation. In Russia, cassiterite deposits are found in the Northeast, Primorye, Yakutia, and Transbaikalia; abroad - in Malaysia, Thailand, Indonesia, China, Bolivia, Nigeria, etc.
Tin Plague
At temperatures below 13.2 °C, the specific volume of pure tin increases by 25.6%, and the metal forms a new modification with semiconductor properties - gray tin (α-Sn), in the crystal lattice of which the atoms are arranged less densely. One modification changes to another the faster the lower the ambient temperature. At −33 °C the transformation rate becomes maximum. The tin cracks and turns to powder. Moreover, the contact of gray tin and white leads to “infection” of the latter. The combination of these phenomena is called the “tin plague.” The scientific study of this phase transition began in 1870 with the work of the St. Petersburg scientist J. Fritzsche. It has been established that this is a process of allotropic transformation of white tin into gray tin with a diamond-type structure. Many valuable observations and thoughts about this process were expressed by D. I. Mendeleev in his “Fundamentals of Chemistry”.
White tin is a silvery-white, shiny metal with a specific tetragonal structure and an s2p2 electronic state - the β phase. Gray tin is a covalent crystal with a diamond structure and an sp3 electronic state - the α phase. Phase transitions of tin from white to gray and back are accompanied by a restructuring of the electronic structure and a strong (26.6%) volume effect. White tin can be supercooled to helium temperatures (phase α-β equilibrium temperature of about +13.2 °C).
There are indications in the literature that tin that gets into a test tube, which once contained a substance capable of infecting, becomes “infected”! It has been shown experimentally that if an InSb crystal is placed on glass for several days (even at room temperature), then after its removal the “memory” of its presence there remains. This glass “contaminates” the white tin sample. But not immediately, but after several days. And not with 100% probability. As the temperature of the glass increases, the “incubation period” sharply increases and the likelihood of “infection” decreases. Holding the seed on the glass at 100 °C completely eliminates the possibility of “contamination.” Rinsing the plate with water, alcohol and other water-absorbing substances also “erases” this “memory”. Loss of “memory” also occurs if the seed was in contact with glass in a vacuum or in a dry desiccator. There is another remarkable phenomenon characteristic of the “tin plague” - this is the “memory” of white tin that it once before turned into gray. J. Fritzsche noticed back in 1870 that white tin, obtained by heating from gray, when cooled again, turns into gray much more easily than during the first time. The sample seems to “remember” its prehistory, and therefore this phenomenon, now widely known, is usually called “memory”. Cohen identified “deterioration” of tin after “recovery” as one of the signs of the “tin plague.”
One way to prevent the "tin plague" is to add a stabilizer, such as bismuth, to the tin.
Interesting facts:
- “Tin plague” is one of the reasons for the death of Scott’s expedition to the South Pole in 1912. It was left without fuel due to the fact that it leaked through tanks sealed with tin, affected by the “tin plague”, so named in 1911 by G. Cohen .
- Some historians point to the “tin plague” as one of the circumstances of the defeat of Napoleon’s army in Russia in 1812 - severe frosts led to the transformation of tin buttons on soldiers’ uniforms into powder.
- The “Tin Plague” destroyed many valuable collections of tin soldiers. For example, in the storerooms of the St. Petersburg Museum of Alexander Suvorov, dozens of figurines turned into dust - in the basement where they were stored, the heating radiators burst in winter.
Story
Tin was known to man already in the 4th millennium BC. e. This metal was inaccessible and expensive, since products made from it are rarely found among Roman and Greek antiquities. There are mentions of tin in the Bible, the Fourth Book of Moses. Tin is (along with copper) one of the components of bronze (see History of copper and bronze), invented at the end or middle of the 3rd millennium BC. e.. Since bronze was the most durable metal and alloy known at that time, tin was a “strategic metal” throughout the “Bronze Age”, more than 2000 years (very approximately: 35-11 centuries BC).