General properties
Steel should not be confused with iron, which is a hard and relatively ductile metal with an atomic diameter of 2.48 angstroms, a melting point of 1535 °C and a boiling point of 2740 °C.
Carbon, on the other hand, is a nonmetal with an atomic diameter of 1.54 angstroms, soft and brittle in most of its allotropes (diamond is the exception). The diffusion of this element in the crystal structure of iron is possible due to the difference in their atomic diameters. As a result of such diffusion, this material is formed. The main difference between iron and steel is the percentage of carbon, which was indicated above. The material may have a different microstructure depending on a particular temperature. It can occur in the following structures (see the iron-carbon phase diagram for more information):
The material retains the properties of iron in its pure state, but the addition of carbon and other elements, both metals and non-metals, improves its physical and chemical properties.
There are many types of steel depending on the elements added to it. The group of carbon steels consists of materials in which carbon is the only additive. Other specialty materials get their names from their basic functions and properties, which are determined by their structure and the additional elements added, such as silicon, cementitious, stainless, structural alloys and so on.
As a rule, all materials with additives are combined under one name - special steels, which differ from ordinary carbon steels, and the latter serve as the base material for the production of special materials. Such diversity of this material in its characteristics and properties has led to the fact that steel began to be called “an alloy of iron and another substance that increases its hardness.”
Metal components
The two main components of steel are found in abundance in nature, which favors its production on a large scale.
The variety of properties and availability of this material makes it suitable for industries such as mechanical engineering, tool making, building construction, contributing to the industrialization of society. Despite its density (the specific gravity of steel kg m3 is 7850, that is, the mass of steel with a volume of 1 m³ is equal to 7850 kilograms, for comparison, the density of aluminum is 2700 kg/m3) it is used in all sectors of industry, including aeronautics. The reasons for its such varied use are both its pliability and at the same time hardness, and its relatively low cost.
Additives and their characteristics
A special classification of steels determines the presence of a specific element in its composition and its percentage by weight. Elements are added to the alloy in order to give it specific properties, for example, increasing its mechanical endurance, hardness, wear resistance, melting ability, and others. Below is a list of the most common additives and the effects they cause.
- Aluminum : added in concentrations close to 1% to increase the hardness of the alloy, and at concentrations less than 0.008% as an antioxidant for heat-resistant materials.
- Boron : at low concentrations (0.001-0.006%) increases the hardenability of the material without reducing its ability to be machined. It is used in low quality materials, for example, in the production of plows and wire, ensuring its hardness and malleability. Also used as nitrogen traps in iron crystal structure.
- Cobalt . Reduces hardenability and leads to strengthening of the material and an increase in its hardness at high temperatures. Also increases magnetic properties. Used in heat-resistant materials.
- Chromium : due to the formation of carbides, it gives steel strength and resistance to high temperatures, increases corrosion resistance, increases the depth of formation of carbides and nitrides during thermochemical processing, is used as a hard stainless coating for axles, pistons, and so on.
- Molybdenum increases hardness and corrosion resistance for austenitic materials.
- Nitrogen is added to facilitate the formation of austenite.
- Nickel makes austenite stable at room temperature, increasing the hardness of the material. Used in heat-resistant alloys.
- Lead forms small globular formations that increase the machinability of steel. This element provides lubrication of the material at a percentage of 0.15% to 0.30%.
- Silicon increases the hardenability and oxidation resistance of the material.
- Titanium stabilizes the alloy at high temperatures and increases its resistance to oxidation.
- Tungsten forms stable and very hard carbides with iron that remain stable at high temperatures, 14-18% of this element creates a cutting steel that can be applied at three times the speed of conventional carbon steel.
- Vanadium increases the material's oxidation resistance and forms complex carbides with iron, which increase fatigue resistance.
- Niobium imparts hardness, ductility and malleability to the alloy. Used in structural materials and automation.
Impurities in the alloy
Impurities are elements that are undesirable in steel.
They are contained in the material itself and enter it as a result of smelting, as they are contained in combustible fuels and minerals. It is necessary to reduce their content, since they deteriorate the properties of the alloy. In the case where their removal from the composition of the material is impossible or expensive, then they try to reduce their percentage to a minimum. Sulfur: its content is limited to 0.04%. The element forms sulfides together with iron, which, in turn, together with austenite form a eutectic with a low melting point. Sulfides are released at grain boundaries. The sulfur content sharply limits the possibility of thermal and mechanical processing of materials at medium and high temperatures, since it leads to destruction of the material along the grain boundaries.
Manganese additives allow you to control the sulfur content of materials. Manganese has a greater affinity with sulfur than iron, so instead of iron sulfide, manganese sulfide is formed, which has a high melting point and good plastic properties. The concentration of manganese must be five times greater than the concentration of sulfur to provide a positive effect. Manganese also increases the machinability of steels.
Phosphorus: the maximum limit for its content in the alloy is 0.04%. Phosphorus is harmful because it dissolves in ferrite, thereby reducing its ductility. Iron phosphide, together with austenite and cementite, forms a brittle eutectic with a relatively low melting point. The release of iron phosphide at grain boundaries makes the material brittle.
Steel sheet weight calculator
Sheet metal is a rolled piece of a specific material, most often steel, that is widely used in industrial production, construction, automotive and other industries.
Sheet steel is the most popular type of sheet blanks, which is produced using cold or hot rolling technology. In the first case, the steel will be called cold-rolled (maximum sheet thickness up to 5 mm), and in the second - hot-rolled.
The KALK.PRO sheet metal weight calculator allows you to calculate the weight of sheet steel based on known thickness and area. You can also familiarize yourself with the metal grade and regulatory documents in the corresponding tabs of the tool. The calculator operates on the basis of GOST 19903-74 “Hot-rolled sheet metal”.
Using the calculator, you can find the weight of rolled sheets of any size and thickness, for example sheets of 1, 3, 6, 8, 10 mm, etc., the standard material is carbon steel St3st with a density of 7850 kg/m 3.
By default, the weight of 1 m 2 of sheet steel is considered.
In order to calculate the weight of sheet metal using our calculator, you must follow the instructions:
- Select the metal type (default is Steel ).
- Confirm the type of assortment - Sheet / Plate .
- Select the metal grade (default Steel St3st ).
- Specify the sheet parameters - thickness t (mm), width a (mm), length b (mm) .
- Enter the quantity of rolled metal, pcs .
Formula for calculating the weight of sheet metal
The weight of sheet metal can also be calculated independently using simple mathematical formulas and tables according to GOST.
Formula for calculating the weight of a metal sheet: m = a × b × t × ρ
Weight table for 1 m 2 sheet metal according to GOST 19903-74
This table applies to hot-rolled steel sheets with a width of 500-4400 mm, thickness from 0.5 to 160 mm.
Source
Specific gravity of metals
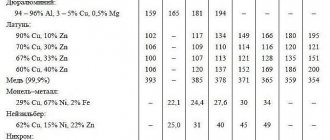
All bodies that have the same volume, but are made from different substances, have different masses, which are directly dependent on its volume. The ratio of the volume of an alloy to its mass—density—is a constant value that will be characteristic of a given substance. And specific gravity is the force of gravity of the volume of a given substance directly taken as a basis. In other words, the specific gravity of a metal is the weight per unit volume of an unconditionally dense (non-porous) material. To indicate the specific gravity, the mass of the dry material should be divided by its volume in a completely dense state. All metals known and used in industry have certain physical and mechanical properties, which, in fact, determine their specific gravity. Metals have characteristic properties, including high strength, thermal and electrical conductivity, ductility. Chemical properties and specific gravity of non-ferrous metals
| Name of non-ferrous metal | Chemical designation | Atomic weight | Melting point, °C | Specific gravity, g/cc |
| Zinc | Zn | 65,37 | 419,5 | 7,13 |
| Aluminum | Al | 26,9815 | 659 | 2,69808 |
| Lead | Pb | 207,19 | 327,4 | 11,337 |
| Tin (Tin) | Sn | 118,69 | 231,9 | 7,29 |
| Copper | Cu | 63,54 | 1083 | 8,93 |
| Titanium | Ti | 47,90 | 1668 | 4,505 |
| Nickel | Ni | 58,71 | 1455 | 8,91 |
| Magnesium (Magnesium) | Mg | 24 | 650 | 1,74 |
| Vanadium | V | 6 | 1900 | 6,11 |
| Tungsten (Wolframium) | W | 184 | 3422 | 19,3 |
| Chrome (Chromium) | Cr | 51,996 | 1765 | 7,19 |
| Molybdenum | Mo | 92 | 2622 | 10,22 |
| Silver (Argentum) | Ag | 107,9 | 1000 | 10,5 |
| Tantalum | Ta | 180 | 3269 | 16,65 |
| Gold (Aurum) | Au | 197 | 1095 | 19,32 |
| Platinum | Pt | 194,8 | 1760 | 21,45 |
Specific gravity of the most common steel grades
| Name (steel type) | Brand or designation | Specific gravity (g/cm3) |
| Stainless steel structural cryogenic | 12Х18Н10Т | 7,9 |
| Stainless steel, corrosion-resistant, heat-resistant | 08Х18Н10Т | 7,9 |
| Low alloy structural steel | 09G2S | 7,85 |
| Quality carbon structural steel | 10,20,30,40 | 7,85 |
| Carbon structural steel | St3sp, St3ps | 7,87 |
| Die tool steel | X12MF | 7,7 |
| Structural spring steel | 65G | 7,85 |
| Die tool steel | 5ХНМ | 7,8 |
| Alloy structural steel | 30ХГСА | 7,85 |
Specific gravity of steel of various grades
| Name (steel type) | Brand or designation | Specific gravity (g/cm3) |
| nickel chrome steel | EI 418 | 8,51 |
| chromium-manganese-nickel steel | Х13Н4Г9 (ЭИ100) | 8,5 |
| chrome steel | 1X13 (EZh1) | 7,75 |
| 2X13 (EZh2) | 7,70 | |
| 3X13 (EZh3) | 7,70 | |
| 4X14 (EZh4) | 7,70 | |
| X17 (EZh17) | 7,70 | |
| X18 (EI229) | 7,75 | |
| X25 (EI181) | 7,55 | |
| X27 (Zh27) | 7,55 | |
| X28 (EZh27) | 7,85 | |
| chromium-nickel steel | 0Х18Н9 (ЭЯ0) | 7,85 |
| 1Х18Н9 (ЭЯ1) | 7,85 | |
| 2Х18Н9 (ЭЯ2) | 7,85 | |
| X17N2 (EI268) | 7,75 | |
| EI307 | 7,7 | |
| EI334 | 8,4 | |
| Х23Н18 (EI417) | 7,9 | |
| chromium-silicon-molybdenum steel | EI107 | 7,62 |
| chromium-nickel-tungsten steel | EI69 | 8,0 |
| chromium-nickel-tungsten with silicon steel | Х25Н20С2 (ЭИ283) | 8,0 |
| chromium-nickel-silicon steel | EI72 | 7,7 |
| other special steel | EI401 | 7,9 |
| EI418 | 8,51 | |
| EI434 | 8,13 | |
| EI435 | 8,51 | |
| EI437 | 8,20 | |
| EI415 | 7,85 |
Specific gravity of carbon and alloy steel
| Name (steel type) | Brand or designation | Specific gravity (g/cm3) |
| high carbon steel | 70 (VS and OVS) | 7,85 |
| medium carbon steel | 45 | 7,85 |
| mild steel | 10 and 10A; 20 and 20A | 7,85 |
| low-carbon electrical (Armco-type iron) steel | A and E; EA; EAA | 7,8 |
| chrome steel | 15ХА | 7,74 |
| chrome-aluminium-molybdenum nitrided steel | 38ХМУА | 7,65 |
| chromium-manganese-silicon steel | 25ХГСА | 7,85 |
| chrome vanadium steel | 30ХГСА | 7,85 |
| 20ХН3А | 7,85 | |
| 40HFA | 7,80 | |
| 50HFA | 7,74 |
How much does one cubic meter of steel weigh?
What do we want to learn today? How much does 1 cube of steel weigh, the weight of 1 m3 of steel?
No problem, you can find out the number of kilograms or the number of tons at once, the mass (weight of one cubic meter, weight of one cube, weight of one cubic meter, weight of 1 m3) is indicated in Table 1. If anyone is interested, you can skim the small text below and read some explanations.
How is the amount of substance, material, liquid or gas we need measured? Except for those cases when it is possible to reduce the calculation of the required quantity to the counting of goods, products, elements in pieces (piece counting), it is easiest for us to determine the required quantity based on volume and weight (mass). In everyday life, the most common unit of volume measurement for us is 1 liter. However, the number of liters suitable for household calculations is not always an applicable way to determine the volume for business activities. In addition, liters in our country have not become a generally accepted “production” and trade unit for measuring volume. One cubic meter, or in its abbreviated version - one cube, turned out to be a fairly convenient and popular unit of volume for practical use. We are accustomed to measuring almost all substances, liquids, materials and even gases in cubic meters. It's really convenient. After all, their costs, prices, rates, consumption rates, tariffs, supply contracts are almost always tied to cubic meters (cubes), and much less often to liters. No less important for practical activities is knowledge of not only the volume, but also the weight (mass) of the substance occupying this volume: in this case we are talking about how much 1 cubic meter weighs (1 cubic meter, 1 cubic meter, 1 m3). Knowing mass and volume gives us a fairly complete idea of quantity. Site visitors, when asking how much 1 cube weighs, often indicate specific units of mass in which they would like to know the answer to the question. As we noticed, most often they want to know the weight of 1 cube (1 cubic meter, 1 cubic meter, 1 m3) in kilograms (kg) or tons (t). Essentially, you need kg/m3 or t/m3. These are closely related units that define quantity. In principle, a fairly simple independent conversion of weight (mass) from tons to kilograms and vice versa is possible: from kilograms to tons. However, as practice has shown, for most site visitors a more convenient option would be to immediately find out how many kilograms 1 cubic (1 m3) of steel weighs or how many tons 1 cubic (1 m3) of steel weighs
, without converting kilograms into tons or vice versa - the number of tons in kilograms per cubic meter (one cubic meter, one cube, one m3). Therefore, in Table 1 we indicated how much 1 cubic meter (1 cubic meter, 1 cubic meter) weighs in kilograms (kg) and tons (t). Choose the table column that you need yourself. By the way, when we ask how much 1 cubic meter (1 m3) weighs, we mean the number of kilograms or the number of tons. However, from a physical point of view, we are interested in density or specific gravity. The mass of a unit volume or the amount of substance contained in a unit volume is bulk density or specific gravity. In this case, the bulk density and specific gravity of steel. Density and specific gravity in physics are usually measured not in kg/m3 or tons/m3, but in grams per cubic centimeter: g/cm3. Therefore, in Table 1, specific gravity and density (synonyms) are indicated in grams per cubic centimeter (g/cm3)