Let's sum it up
Their chemical properties depend on the activity of metals. Simple substances - metals - are reducing agents in redox reactions. By the position of a metal in the electrochemical series, one can judge how actively it is capable of entering into chemical reactions (i.e., how strongly the metal exhibits reducing properties).
Finally, we’ll share a table that will help you remember what metals react with and prepare for your chemistry test.
Properties of metals and alloys: mechanical, physical, chemical
Question
Mechanical properties
The main mechanical properties include: - strength - ductility - hardness
Strength is the ability of a material to resist destruction under loads. Plasticity is the ability of a material to change its shape and size under the influence of external forces. Hardness is the ability of a material to resist the penetration of another body into it.
Physical properties
Physical properties include: - color - density - melting point - thermal conductivity - electrical conductivity - magnetic properties
Color is the ability of metals to reflect radiation of a certain wavelength. For example, copper is pinkish-red in color, while aluminum is silvery-white.
The density of a metal is determined by the ratio of mass to unit volume. Based on their density, metals are divided into light (less than 4500 kg/m3) and heavy.
Melting point is the temperature at which a metal changes from solid to liquid. Based on the melting point, they distinguish between refractory (tungsten - 3416 °C, tantalum - 2950 °C, etc.) and low-melting (tin - 232 °C, lead - 327 °C). In SI units, the melting point is expressed in degrees Kelvin (K).
Thermal conductivity is the ability of metals to transfer heat from more heated areas of the body to less heated ones. Silver, copper, and aluminum have high thermal conductivity. In SI units, thermal conductivity has the dimension W/(m K).
The ability of metals to conduct electric current is assessed by two opposing characteristics - electrical conductivity and electrical resistance. Electrical conductivity is measured in SI units in siemens (Sm). Electrical resistance is expressed in ohms (Ohms). Good electrical conductivity is necessary, for example, for current-carrying wires (they are made of copper, aluminum). In the manufacture of electric heating devices and furnaces, alloys with high electrical resistance (from nichrome, constantan, manganin) are required. As the temperature of a metal increases, its electrical conductivity decreases, and as it decreases, it increases. Magnetic properties are expressed in the ability of metals to be magnetized. Iron, nickel, cobalt and their alloys, which are called ferromagnetic, have high magnetic properties. Materials with magnetic properties are used in electrical equipment and for the manufacture of magnets.
Chemical properties
Chemical properties characterize the ability of metals and alloys to resist oxidation or combine with various substances: atmospheric oxygen, acid solutions, alkali solutions, etc.
Chemical properties include: - corrosion resistance - heat resistance
Corrosion resistance is the ability of metals to resist chemical destruction under the influence of an external aggressive environment on their surface (corrosion occurs when it enters into chemical interaction with other elements).
Heat resistance - the ability of metals to resist oxidation at high temperatures
Chemical properties are taken into account primarily for products or parts operating in chemically aggressive environments: - containers for transporting chemical reagents - chemical pipelines - instruments and instruments in the chemical industry
Technological properties characterize the ability of a material to be subjected to various methods of cold and hot processing.
1. Casting properties - characterize the ability of a material to produce high-quality castings from it. Fluidity – characterizes the ability of molten metal to fill a casting mold. Shrinkage (linear and volumetric) – characterizes the ability of a material to change its linear dimensions and volume during the process of solidification and cooling. To prevent linear shrinkage when creating models, non-standard meters are used. Liquation is the heterogeneity of the chemical composition by volume.
2. The ability of a material to be processed by pressure is the ability of a material to change size and shape under the influence of external loads without collapsing. It is controlled as a result of technological tests carried out under conditions as close as possible to production ones. The sheet material is tested for bending and stretching of the spherical dimple. The wire is tested for bending, twisting, and winding. Pipes are tested for expansion, flattening to a certain height and bending. The criterion for the suitability of the material is the absence of defects after testing.
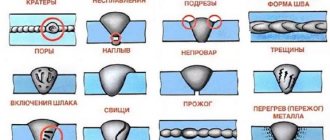
3. Weldability is the ability of a material to form permanent joints of the required quality. Evaluated by the quality of the weld.
4. Cutting ability - characterizes the ability of a material to be processed by various cutting tools. It is assessed by tool life and the quality of the surface layer.
Performance properties characterize the ability of a material to work under specific conditions.
1. Wear resistance - the ability of a material to resist surface destruction under the influence of external friction.
2. Corrosion resistance (see Electrochemical and chemical corrosion of metals) – the ability of a material to resist the action of aggressive acidic and alkaline environments.
3. Heat resistance (see Heat resistance. Heat-resistant steel. Heat-resistant alloys.) is the ability of a material to resist oxidation in a gas environment at high temperatures.
4. Heat resistance is the ability of a material to retain its properties at high temperatures.
5. Cold resistance - the ability of a material to retain plastic properties at subzero temperatures.
6. Antifriction - the ability of a material to be worn in to another material.
Question
| Crystallization of metals and alloys |
| Crystallization process. When a metal changes from a liquid to a solid state, crystals form. This process is called crystallization.
|
Question
Mechanical properties
The main mechanical properties include: - strength - ductility - hardness
Strength is the ability of a material to resist destruction under loads. Plasticity is the ability of a material to change its shape and size under the influence of external forces. Hardness is the ability of a material to resist the penetration of another body into it.
Physical properties
Physical properties include: - color - density - melting point - thermal conductivity - electrical conductivity - magnetic properties
Color is the ability of metals to reflect radiation of a certain wavelength. For example, copper is pinkish-red in color, while aluminum is silvery-white.
The density of a metal is determined by the ratio of mass to unit volume. Based on their density, metals are divided into light (less than 4500 kg/m3) and heavy.
Melting point is the temperature at which a metal changes from solid to liquid. Based on the melting point, they distinguish between refractory (tungsten - 3416 °C, tantalum - 2950 °C, etc.) and low-melting (tin - 232 °C, lead - 327 °C). In SI units, the melting point is expressed in degrees Kelvin (K).
Thermal conductivity is the ability of metals to transfer heat from more heated areas of the body to less heated ones. Silver, copper, and aluminum have high thermal conductivity. In SI units, thermal conductivity has the dimension W/(m K).
The ability of metals to conduct electric current is assessed by two opposing characteristics - electrical conductivity and electrical resistance. Electrical conductivity is measured in SI units in siemens (Sm). Electrical resistance is expressed in ohms (Ohms). Good electrical conductivity is necessary, for example, for current-carrying wires (they are made of copper, aluminum). In the manufacture of electric heating devices and furnaces, alloys with high electrical resistance (from nichrome, constantan, manganin) are required. As the temperature of a metal increases, its electrical conductivity decreases, and as it decreases, it increases. Magnetic properties are expressed in the ability of metals to be magnetized. Iron, nickel, cobalt and their alloys, which are called ferromagnetic, have high magnetic properties. Materials with magnetic properties are used in electrical equipment and for the manufacture of magnets.
Chemical properties
Chemical properties characterize the ability of metals and alloys to resist oxidation or combine with various substances: atmospheric oxygen, acid solutions, alkali solutions, etc.
Chemical properties include: - corrosion resistance - heat resistance
Corrosion resistance is the ability of metals to resist chemical destruction under the influence of an external aggressive environment on their surface (corrosion occurs when it enters into chemical interaction with other elements).
Heat resistance - the ability of metals to resist oxidation at high temperatures
Chemical properties are taken into account primarily for products or parts operating in chemically aggressive environments: - containers for transporting chemical reagents - chemical pipelines - instruments and instruments in the chemical industry
Technological properties characterize the ability of a material to be subjected to various methods of cold and hot processing.
1. Casting properties - characterize the ability of a material to produce high-quality castings from it. Fluidity – characterizes the ability of molten metal to fill a casting mold. Shrinkage (linear and volumetric) – characterizes the ability of a material to change its linear dimensions and volume during the process of solidification and cooling. To prevent linear shrinkage when creating models, non-standard meters are used. Liquation is the heterogeneity of the chemical composition by volume.
2. The ability of a material to be processed by pressure is the ability of a material to change size and shape under the influence of external loads without collapsing. It is controlled as a result of technological tests carried out under conditions as close as possible to production ones. The sheet material is tested for bending and stretching of the spherical dimple. The wire is tested for bending, twisting, and winding. Pipes are tested for expansion, flattening to a certain height and bending. The criterion for the suitability of the material is the absence of defects after testing.
3. Weldability is the ability of a material to form permanent joints of the required quality. Evaluated by the quality of the weld.
4. Cutting ability - characterizes the ability of a material to be processed by various cutting tools. It is assessed by tool life and the quality of the surface layer.
Performance properties characterize the ability of a material to work under specific conditions.
1. Wear resistance - the ability of a material to resist surface destruction under the influence of external friction.
2. Corrosion resistance (see Electrochemical and chemical corrosion of metals) – the ability of a material to resist the action of aggressive acidic and alkaline environments.
3. Heat resistance (see Heat resistance. Heat-resistant steel. Heat-resistant alloys.) is the ability of a material to resist oxidation in a gas environment at high temperatures.
4. Heat resistance is the ability of a material to retain its properties at high temperatures.
5. Cold resistance - the ability of a material to retain plastic properties at subzero temperatures.
6. Antifriction - the ability of a material to be worn in to another material.
Question
| Crystallization of metals and alloys |
| Crystallization process. When a metal changes from a liquid to a solid state, crystals form. This process is called crystallization. The process of metal crystallization can be viewed from cooling curves, which are usually obtained experimentally. For example, for a pure metal that is cooled very slowly, the cooling curve shows that if the metal is in a liquid state, the temperature decreases almost uniformly. If the metal is cooled to the melting temperature Tmelt ( point a on the curve), then crystallization begins and the temperature drop stops, despite the continuous transfer of heat to the surrounding atmosphere. The resulting horizontal section on the cooling curve shows that the process of formation of crystals with the release of heat occurs in the metal, called the heat of crystallization. Crystallization proceeds from point a to point b, where it ends and the metal solidifies. A further drop in temperature on the curve indicates cooling of the solidified ingot (Fig. A). In metal alloys, the cooling curve has a slightly different form. Having cooled to the melting temperature Tpl , the alloy remains liquid for some time. Crystallization of the alloy begins at the supercooling temperature Tp, which lies below the theoretical melting temperature. The difference between the theoretical and actual crystallization temperatures is called the degree of supercooling. It depends on the nature of the alloy, its purity and cooling rate. The greater the cooling rate of the alloy, the greater the degree of supercooling. The loop in the cooling curve shows that crystallization is accompanied by the release of heat, which raises the temperature of the alloy to the melting temperature, maintaining it until the metal completely solidifies. (Fig. B) Amorphous bodies solidify gradually. In this case, the cooling curve will be smooth, without horizontal areas. (Fig. B) The process of crystal formation consists of two simultaneous stages: the appearance of nuclei - stable centers of crystallization and the growth of crystals around these centers. At first, each crystal in the liquid grows freely , maintaining the correct geometric shape. Since many crystalline centers are formed at the same time and the growth of crystals occurs in all directions, adjacent crystals, growing, begin to directly touch each other and their correct shape is disrupted. As a result, the crystal takes on a rounded, grain-like shape. Such crystals are usually called crystallites or grains. Depending on the solidification conditions, the grains can be large, clearly visible to the naked eye, or small, which can only be seen using a metallographic microscope. The crystallization process can be described quantitatively if the nucleation of crystallization centers and the rate of crystal growth are known. The number of crystallization centers and the growth rate of crystals depend on the degree of supercooling of the metal. With an increase in the degree of supercooling ∆T, the number of centers and the growth rate also increase, reaching a maximum value. However, the nature of the growth in the number of centers and the growth rate is different. If the degree of supercooling is small, then the growth rate prevails over the number of centers, resulting in the formation of a coarse-grained structure. With an increase in the degree of supercooling, the growth rate does not change, the number of centers continues to increase, which leads to the formation of a fine-grained structure. |