Physical properties of metals
Metals are chemical elements whose atoms tend to give up electrons during a reaction. They have a metallic crystal lattice and general physical properties. Currently, more than 87 metals are known.
Metals are characterized by a number of properties:
- hardness (except mercury, which is a liquid);
- metallic shine;
- conductivity of electric current and heat;
- plastic.
Metals do not collapse upon impact, but change shape. This feature is associated with the fact that they are used to produce wire, metal sheets, etc. The development of the Bronze and Iron Ages is associated with the production of goods from metals.
What physical characteristics are we talking about?
Density is a quantity that characterizes the amount of a substance present in a known volume. According to this definition, it can be calculated mathematically as follows:
ρ = m/V.
This quantity is denoted by the Greek letter ρ (rho).
Density is a universal characteristic because it can be used to compare different materials. This fact can be used to identify them, which is what the Greek philosopher Archimedes did, according to legend (he was able to identify the fake gold crown by measuring the value of ρ for it).
This parameter for a specific material depends on two main factors:
- on the mass of the atoms and molecules that make up the substance;
- on average interatomic and intermolecular distances.
For example, any of the transition metals (gold, iron, vanadium, tungsten) has a higher density than any carbon material, since the mass of an atom of the latter is tens of times less. Another example. Graphite and diamond are two carbon structures. The second one is more dense because the interatomic distances in its lattice are smaller.
Physical properties of non-metals
Nonmetals are chemical elements whose atoms tend to accept foreign electrons. They are characterized by atomic and molecular crystal lattices. Non-metal atoms do not have general physical properties. There are currently 22 nonmetals.
Nonmetals are characterized by a number of properties:
- fragility (non-metals cannot be forged);
- lack of shine;
- non-conductivity of electric current and heat.
The location of metals and non-metals in the periodic table D.I. Mendeleev
You can determine whether a simple substance is a metal or a non-metal using the periodic table. Metals are located below the hydrogen-boron-silicon-arsenic-tellurium-astatine diagonal, and non-metals are located above.
Red cells are non-metals, blue cells are metals
Elements located near the diagonal have mixed properties: they exhibit both metallic and non-metallic properties. They are called semimetals.
Red cells are semimetals
Semimetals have a covalent crystal lattice with metallic conductivity (electrical conductivity). They either do not have enough valence electrons to form a full-fledged covalent bond, or they are not held tightly enough due to the large size of the atom. Therefore, the bond in covalent crystals of these elements is partially metallic in nature.
Patterns in the table D.I. Mendeleev
Each atom is made up of protons, neutrons and electrons. Protons and neutrons are found in the nucleus, which carries a positive charge. Negatively charged electrons move around the nucleus. The atomic number indicates the number of protons.
The greater the charge of the nucleus, the more electrons are attracted to it. Thus, it is more difficult for an atom to give up electrons. Therefore, in a period from left to right, with increasing serial number, metallic properties weaken, and non-metallic properties increase.
Nonmetals tend to accept electrons from other atoms. The period in the table indicates the number of electronic levels. As the number of orbitals increases, electrons move away from the nucleus and it becomes more difficult for the atom to retain electrons in the final levels. Thus, in a group from top to bottom, the number of orbitals increases, so metallic properties increase, and non-metallic properties decrease.
Density of steel
The first mentions of steel are contained in Indian sources dating back to approximately 1 millennium BC. e. Steel swords made by Indian craftsmen were stronger and sharper than bronze ones. Steel was processed in the Middle East and Ancient Rome. It was steel swords and armor that helped the Roman legions in their victorious march through the ancient world.
The rebirth of the material occurred in the 19th century, when the open-hearth method of its smelting was developed, which made it possible to obtain alloys of high and stable quality in large quantities. In the 20th century, steel became the main structural material. One of the important characteristics of any material is its density - the mass of a substance per unit volume.
Density of steel
Density is measured in grams per cubic centimeter or tons per cubic meter. The digital density value for these two units of measurement will be the same. The density of the same material at different temperatures changes due to the phenomenon of thermal and volumetric expansion. For most substances, including metals, density decreases with increasing temperature.
Density of structural alloy steel
Structural alloy alloys are used in the production of highly loaded critical structures, including those operating in aggressive environments. The density of grade 30KhGSA is close to the standard value of 7.85 t/m3, the density of low-alloy structural steel for welded structures
Low-alloy alloys have excellent weldability and high corrosion resistance, so they are widely used for critical structures in construction and shipbuilding. The HC of steel in this group ranges from 7.85-7.87 t/m3 and is given in the table:
| Group | Brand | Density |
| low alloy structural | 09G2S | 7,85 |
| high carbon | 70 (VS and OVS) | 7,85 |
| medium carbon | 45 | 7,85 |
| low carbon | 10, 10A, 20, 20A | 7,85 |
| carbon structural | St3sp, St3ps | 7,87 |
Density of structural steel with increased machinability
The specific gravity of 30KhGSA steel used for shafts, axles, and levers is 7.85 t/m3. When heated to 200 ºС it decreases to 7.8. The density of structural bearing steel grade 35ХГ2 is 7.8 t/m3.
The specific gravity of steel 12Х2Н4А, used to create highly loaded gears, piston pins, etc., is 7.84 t/m3 at 20 ºС and decreases to 7.63 when heated to 600 ºС
Density of structural spring steel
Spring-spring alloys have increased elasticity while maintaining high strength and are used for the manufacture of elastic elements of mechanisms - springs, springs, shock absorbers. The density of grade 65G is 7.85 t/m3.
Density of structural carbon quality steel
High-quality structural carbon steel grades 10, 20, 30, 40 has a density of 7.85 t/m3
Density of stainless steel
The density of a substance is calculated by dividing the mass of an object by its volume. Such calculations have already been made for all substances known to man, and metrological services periodically repeat and refine these measurements. In practice, people face another practical task: knowing the material from which the product is made, determine its mass.
The density of a substance is also called specific gravity (or, in everyday life, specific gravity) - that is, the mass of a solid physical body made of a given substance and having a unit volume.
Stainless steel
It should be noted that when using the term “mass”, in 99% of cases people are dealing with weight - the force of attraction of the physical body to the Earth.
The fact is that to determine body weight in a strict physical sense, sophisticated equipment is required, available only in the largest scientific centers.
For practical use, in most cases, conventional, more or less accurate scales using the Earth's gravity and springs, or levers and standard weights, or piezoelements are sufficient.
In practice, to calculate the weight of a linear or square meter of rolled metal, the specific gravity, or density of the material from which it is made, is used. In reference books on the assortment of rolled metal, among the main characteristics of each grade, the mass of a linear or square meter and the density value used in the calculations must be indicated.
In most cases, calculations based on the mass of a linear or square meter are sufficient for practical applications. Raw materials and components are purchased with a certain standardized stock, and before shipment to the consumer, the product is weighed on scales for accurate settlements between contractors.
However, you need to understand that the data in the directory is calculated based on the standard density of steel, most often it is 7.85 t/m3. At the same time, the actual density of a particular steel grade depends on the composition and specific amount of additives and can range from 7.6 to 8.8 t/m3.
This can give an error of up to 10% up or down for a product made from a very light or, conversely, very heavy alloy. For a small amount of metal the difference will be small and can be neglected. However, for complex products that use large volumes of metal, more accurate calculations will be required.
The mass will be needed when creating an application for the purchase of metal. Based on the density of a given alloy, an adjustment is made to the reference values of the mass of one linear or square meter, and then the already specified value is used in the calculations.
How to calculate P or perform 1 meter mass adjustment?
The practical method for determining density is quite simple and is known to us from a school physics course. A sample of material is lowered into a measuring container filled with water to a certain level. The water level rises to a certain height. The volume of displaced water is equal to the volume of the sample. The mass of the sample is determined by weighing on an accurate balance. Density will be equal to the ratio of mass and volume.
To adjust the mass of a linear or square meter, you need to divide the value from the reference book by the density from the reference book and multiply the result by the measured density of the sample material. The corrected value will be obtained.
If similar calculations are expected to be repeated, then it will be more convenient to calculate a correction factor equal to the ratio of the standard density and the density of the sample, and then apply it in the calculations.
Density of 12Х18Н10Т and some stainless steels
Grade 12×18N10T is one of the most widely used stainless steels. The density for it and several popular brands in production is given in the table, the brands are arranged in order of increasing density. The third column shows the density adjustment factor relative to the standard value of 7.85:
| steel grade | Density t/m3 | Correction factor |
| 08Х22Н6Т15Х28 | 7,60 | 0,97 |
| 08Х1312Х17 | 7,70 | 0,98 |
| 04Х18Н1008Х18Н12Б12Х18Н10Т17Х18Н9 | 7,90 | 1,01 |
| 08Х18Н12Т10Х23Н18 | 7,95 | 1,01 |
| 06ХН28МДТ08ХН28МДТ | 7,96 | 1,01 |
| 10Х17Н13М2Т | 8,00 | 1,02 |
| 08Х17Н15М3Т | 8,10 | 1,03 |
Density of other steels and alloys
The specific gravity of steel of other groups is given in the table:
| Steel type | Brand | Density |
| cryogenic stainless structural | 12Х18Н10Т | 7,9 |
| heat-resistant stainless steel corrosion-resistant | 08Х18Н10Т | 7,9 |
| stamped instrumental | X12MF | 7,7 |
| stamped instrumental | 5ХНМ | 7,8 |
| low-carbon electrical (Armco) | A and E; EA; EAA | 7,8 |
| chromium | 15ХА | 7,74 |
| chrome-aluminium-molybdenum nitrided | 38ХМУА | 7,71 |
| chromium-manganese-silicon | 25ХГСА | 7,85 |
| chrome vanadium | 30ХГСА; 20ХН3А | 7,85 |
Steel - concept and its characteristics
Steel is the most common material for the manufacture of structures, parts, mechanisms and tools.
Steels include all alloys of iron and carbon, and the share of iron must be at least 45%, and the share of carbon - less than 2.14 percent.
Carbon, lining up in the molecular structures of iron, increases strength and hardness, but makes the alloy less ductile and malleable. In addition to carbon, the alloy contains metals and non-metals.
The most important characteristics of the alloy include:
- shear modulus;
- elastic modulus;
- density;
- linear expansion coefficient.
Different areas of application of materials require them to have different physical and chemical properties. For example, steel alloys with a high modulus of elasticity are used for the production of springs and spring-type shock absorbers. These properties are purposefully changed as a result of the addition of various additives.
Melting steel
The density of steel, or HC steel, is one of the most important characteristics of the alloy. Based on it, the designer calculates the weight of the part and the total weight of the product, logistics organizes the purchase and delivery of raw materials, blanks and finished products, economists determine the cost. The weight of steel is determined as the product of density and volume.
Steel classification
Depending on the proportion of non-metallic impurities determined by the method of smelting a given grade, steel alloys are divided into:
- especially high quality;
- high quality;
- ordinary quality.
Based on their chemical composition, alloys are also divided into alloyed and carbon.
Carbon steels
Used primarily for the production of welded structures and contains from 0.25 to 2.14 percent carbon. Within the group, they are further divided into subgroups, and also according to the percentage of carbon:
- high carbon (0.6-2.14);
- medium carbon (0.3-0.55);
- low carbon (below 0.25).
They also contain silicon and manganese as additives. In addition to useful, purposefully introduced additives, the alloy may also contain harmful impurities that negatively affect its physicochemical properties:
- phosphorus reduces ductility when heated and increases brittleness when cooled;
- sulfur leads to the formation of microcracks.
Low carbon steel
Other impurities may also be present in the alloy.
Alloy steel
In order for the alloy to acquire the required properties during melting, useful additives or alloying elements are added to it, most often metals such as aluminum, molybdenum, chromium, manganese, nickel, vanadium and others.
The properties of the alloy change quite significantly: the alloy acquires resistance to corrosion, special strength, high malleability, increased or decreased electrical conductivity, etc. An alloy with such additives is called alloy steel.
Based on the percentage of alloying additives, they are divided into three groups:
- highly alloyed – over 11;
- medium alloyed – from 4 to 11;
- low alloy – less than 4.
By area of application, steel alloys are divided into:
- tool - high-strength alloys are used for the manufacture of tools, dies, cutters, drills and cutters;
- structural - used for the production of bodies and components of vehicles, machine tools, building structures;
- special. This group includes alloys with increased resistance to acidic and alkaline environments, radiation, stainless alloys, electrical materials, etc.
Alloy steel
Some additives and treatments increase the density of the material, while others reduce it, for example:
| Processing method or additive | Density change |
| carbon | is decreasing |
| chrome, aluminum, manganese | is decreasing |
| cobalt, tungsten, copper | growing |
| drawing | grows within three percent |
, please select a piece of text and press Ctrl+Enter.
Chemical properties of metals
All metals exhibit reducing properties. The ease of donating an external electron is used in photovoltaic cells. The degree of activity is determined by the activity series. The most active ones have one electron at the outer level.
The general chemical properties of metals are expressed in reactions with the following compounds.
- With non-metals
4 Li + O2→ 2 LiO2
3 Mg + N2 → Mg3N2
Reactive metals react with halogens and oxygen. Only lithium, calcium and magnesium interact with nitrogen. Most metals, when interacting with oxygen, form oxides, and the most active metals form peroxides (N2O2).
- With metal oxides
2 Ca + MnO2 → 2 CaO + Mn(heating)
- With acids
Mg + H2SO4(dil)→MgSO4 + H2
Hydrogen in acids is replaced only by those metals that come before hydrogen in the voltage series.
- With salt solutions
Fe + CuSO4→ Cu + FeSO4
Cu + 2 AgNO3→ 2 Ag + Cu(NO3)2
More active metals displace less active metals from compounds.
- Chemical properties of alkali and alkaline earth metals (reactions with water)
2 Na + 2 H2O → 2 NaOH + H2
Ca + 2 H2O →Ca(OH)2 + H2
Finding metals in nature
Noble low-active metals, in a free (native) state, are not often found in nature due to their rarity. Gold, silver, platinum, and copper are found in the native state.
Metals are found in small quantities in sea water (1.05%, - 0.12%), plants, and living organisms. They play an important role in living organisms. For example, they are part of essential nutrients and enzymes necessary for the operation of many processes.
Perhaps the most important role in the body of living beings is played by iron, which is part of the blood, or more precisely, a component of hemoglobin in the blood - heme. Hemoglobin is a blood protein that transports oxygen to the necessary organs of a living organism through the blood. Iron deficiency causes anemia, a disease characterized by lethargy, weakness, decreased performance and fatigue, and in some cases has fatal consequences. The disease is determined primarily by a blood test.
Table of contents of some metals in the earth's crust
Uranium ore
Uranium is commonly used in the nuclear industry as fuel. But the element is very active, so uranium is not found in its pure form; formations such as uranium ores are used for its production. The latter contains enough of the element to make mining the ore economically viable.
Uranium ore
Alloys
Aluminum alloys
Aluminum alloys are very often used in industries. This is due to the fact that aluminum alloys are highly durable.
The most used aluminum alloys: duralumin, silumin.
METALLOTHERMY is a method of reducing metals and compounds of these metals. Accompanied by the release of heat. This process is subject to classification according to the nature of the reducing metal (aluminothermy, magnesiumothermy, calcithermy, etc.).
Conclusion
Metals are a very large category of substances, combining elements with different physical and chemical properties. But at the same time, they have many things in common, the main one of which is accepting the role of a reducing agent in reactions, donating their electrons. Also, many metals are widely used by mankind in industry, in everyday life, in medicine, in pharmaceuticals, etc. Historically, it was the extraction of iron from ores that marked a new round of development in human history. Therefore, it is difficult to overestimate this category of substances.
Modern humanity uses metals in industry, in everyday life and in the household. For many centuries, metal has been a faithful companion of progress. But metal has become most widespread in construction, making buildings stronger and construction times much shorter.
Chemical properties of non-metals
Nonmetals exhibit oxidizing properties. The most active nonmetal is fluorine. It reacts violently with all substances, and some reactions are accompanied by combustion and explosion. Even water and platinum burn in a fluorine atmosphere. Fluorine oxidizes oxygen and forms oxygen fluoride OF2.
Nonmetals react with the following substances.
- With metals
3 F + 2 Al → 2 AlF3 (heating)
S + Fe →FeS (heating)
- With other non-metals
2 F2 + C → CF2 (heating)
S + O2→ SO2(heating)
- With complex substances
4 F2 + CH4→CH3F + HF
3 O2 + 4 NH3→ 2 N2 + 6 H2O
Non-metals such as boron, graphite, and diamond have less activity. They may exhibit restorative properties.
2 C + MnO2 → Mn + 2 CO
4 H2 + Fe3O2 → 3 Fe + 4 H2O
What is specific gravity
Specific gravity is the density multiplied by the acceleration of gravity (gravity) or the ratio of the weight of a body to its volume. It is unacceptable to confuse it with density. However, this often occurs due to confusion between the concepts of mass and weight. The weight of a body, and therefore its specific gravity, changes depending on the force of gravity. It is not a constant value. Depending on the place where the item is located, it has different meanings. This physical quantity will be different even at different points on the Earth. The acceleration of gravity at the equator is greater than at the poles. Mass and density are constant.
For example, you can calculate the specific gravity of silver. On Earth, this value will be 10,500 kg/m³ (density of pure metal). Multiplying by 9.81 m/s2 (gravity), you can get 103005 N/m³. And on the Moon, 10,500 kg/m³ is multiplied by 1.62 m/s2 (gravity on the Moon). The result is different - 17.01 N/m³. In the cabin of a ship rotating around the Earth there is weightlessness and acceleration is zero. Consequently, the weight of any material here is zero.
All values will be different. The greatest value will be in the first case, because on Earth the acceleration of gravity has the greatest significance. In zero gravity, a thing weighs nothing. The density of the same material will be the same anywhere. It is a constant.
In order to create tables of the specific gravity of metals on various planets (or in other conditions), it is necessary to know the acceleration of gravity and density.
Corrosion of metal
Corrosion is the process of destruction of metals or metal structures under the influence of oxygen, water and harmful impurities. Not all metals corrode. Their durability depends on a number of factors.
- No corrosion occurs on noble metals.
- On the surface of aluminum, titanium, zinc, chromium and nickel there is an oxide film that prevents corrosion processes.
There are several types of corrosion – chemical and electrochemical.
Chemical corrosion
Chemical corrosion is accompanied by chemical reactions. It is formed under the influence of gases.
3 Fe + 2 O2 → Fe3O4
2 Fe + 3 Cl2 → 2 FeCl3
Electrochemical corrosion
Electrochemical corrosion is the process of destruction of metals or metal structures, which is accompanied by electrochemical reactions. Most metals contain impurities. During the corrosion process, not only metals, but also metal impurities can serve as electrodes.
For example, iron may contain tin impurities. In this case, at the anode, electrons are transferred from tin to iron and the metals dissolve, i.e. iron is subject to corrosion. At the cathode, hydrogen is reduced from water or dissolved oxygen. Electrochemical corrosion can be accompanied by the following processes.
Anode: Fe2+ – 2e → Fe0
Cathode: 2H+ + 2e → H2
Methods of protection against corrosion
Various methods of protecting metals from corrosion are popular in industry.
- Protective coatings
Coatings protect surfaces from oxidizing agents. They are various substances:
- coating with a less active metal (iron is coated with tin);
- paints, varnishes, lubricants.
- Creation of special alloys
The physical properties of alloys and pure metals are different. Therefore, to increase the resistance, additional metals must be added to the alloy.
How do you find the value?
The density of metals is a characteristic that can be determined in two fundamentally different ways:
- experimental;
- theoretical.
Experimental methods are of the following types:
- Direct measurements of body weight and volume. The latter is easy to calculate if the geometric parameters of the body are known and its shape is ideal, for example, a prism, pyramid or ball.
- Hydrostatic measurements. In this case, special scales are used, invented by Galileo in the 16th century. The principle of their operation is quite simple: first, a body of unknown density is weighed in air, and then in liquid (water). After this, the required value is calculated using a simple formula.
As for the theoretical method of determining the density of metals, this is a fairly simple method that requires knowledge of the type of crystal lattice, the interatomic distance in it and the mass of the atom. Next, using the example of osmium, we will show how this method is used.
Biological role of metals and non-metals
Organisms contain many different metals and non-metals. Various chemical elements in the body may not be enough, so you have to consume them from the outside. Chemical elements can be divided into two large groups - macroelements and microelements.
Macroelements include substances whose content in the body exceeds 0.005%. This group includes hydrogen, carbon, oxygen, nitrogen, sodium, magnesium, phosphorus, sulfur, chlorine, potassium, calcium. Microelements are elements whose content does not exceed 0.005%. These include iron, copper, selenium, iodine, chromium, zinc, fluorine, manganese, cobalt, molybdenum, silicon, bromine, vanadium, boron. Each macro- and microelement in the body performs a specific function.
How to determine the density of a metal - Metalist's Handbook
The first mentions of steel are contained in Indian sources dating back to approximately 1 millennium BC. e.
Steel swords made by Indian craftsmen were stronger and sharper than bronze ones. Steel was processed in the Middle East and Ancient Rome.
It was steel swords and armor that helped the Roman legions in their victorious march through the ancient world.
The rebirth of the material occurred in the 19th century, when the open-hearth method of its smelting was developed, which made it possible to obtain alloys of high and stable quality in large quantities. In the 20th century, steel became the main structural material. One of the important characteristics of any material is its density - the mass of a substance per unit volume.
Density of steel
Density is measured in grams per cubic centimeter or tons per cubic meter. The digital density value for these two units of measurement will be the same.
The density of the same material at different temperatures changes due to the phenomenon of thermal and volumetric expansion.
For most substances, including metals, density decreases with increasing temperature.
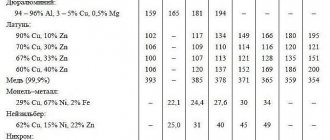
| All metals have certain physical and mechanical properties, which, in fact, determine their specific gravity. To determine how suitable a particular alloy of ferrous or stainless steel is for production, the specific gravity of rolled metal is calculated. All metal products that have the same volume, but are made from different metals, for example, iron, brass or aluminum, have different mass, which is directly dependent on its volume. In other words, the ratio of the volume of the alloy to its mass—specific density (kg/m3)—is a constant value that will be characteristic of a given substance. The density of the alloy is calculated using a special formula and is directly related to the calculation of the specific gravity of the metal. The specific gravity of a metal is the ratio of the weight of a homogeneous body of this substance to the volume of the metal, i.e. this is density, in reference books it is measured in kg/m3 or g/cm3. From here you can calculate the formula for finding out the weight of a metal. To find this you need to multiply the reference density value by the volume. The table shows the densities of non-ferrous metals and ferrous iron. The table is divided into groups of metals and alloys, where under each name the grade according to GOST and the corresponding density in g/cm3 are indicated, depending on the melting point. To determine the physical value of specific density in kg/m3, you need to multiply the tabulated value in g/cm3 by 1000. For example, this way you can find out what the density of iron is - 7850 kg/m3.
Ferrous metals in the table include iron, manganese, titanium, nickel, chromium, vanadium, tungsten, molybdenum, and ferrous alloys based on them, for example, stainless steel (density 7.7-8.0 g/cm3), black steel ( density 7.85 g/cm3) is mainly used by manufacturers of metal structures in Ukraine, cast iron (density 7.0-7.3 g/cm3). The remaining metals are considered non-ferrous, as well as alloys based on them. Non-ferrous metals in the table include the following types: − light – magnesium, aluminum; − noble metals (precious) - platinum, gold, silver and semi-precious copper; − low-melting metals – zinc, tin, lead. Specific gravity of non-ferrous metals | Atomic weight | Melting point, °C | Specific gravity, g/cc |
| Zinc Zn (Zinc) | 65,37 | 419,5 | 7,13 |
| Aluminum Al | 26,9815 | 659 | 2,69808 |
| Lead Pb (Lead) | 207,19 | 327,4 | 11,337 |
| Tin Sn (Tin) | 118,69 | 231,9 | 7,29 |
| Copper Cu (Copper) | 63,54 | 1083 | 8,96 |
| Titanium Ti (Titanium) | 47,90 | 1668 | 4,505 |
| Nickel Ni (Nickel) | 58,71 | 1455 | 8,91 |
| Magnesium Mg (Magnesium) | 24 | 650 | 1,74 |
| Vanadium V | 6 | 1900 | 6,11 |
| Tungsten W (Wolframium) | 184 | 3422 | 19,3 |
| Chrome Cr (Chromium) | 51,996 | 1765 | 7,19 |
| Molybdenum Mo (Molybdaenum) | 92 | 2622 | 10,22 |
| Silver Ag (Argentum) | 107,9 | 1000 | 10,5 |
| Tantalum Ta (Tantal) | 180 | 3269 | 16,65 |
| Iron Fe (Iron) | 55,85 | 1535 | 7,85 |
| Gold Au (Aurum) | 197 | 1095 | 19,32 |
| Platinum Pt (Platina) | 194,8 | 1760 | 21,45 |
When rolling non-ferrous metal blanks, it is also necessary to know exactly their chemical composition, since their physical properties depend on it.
For example, if aluminum contains impurities (even within 1%) of silicon or iron, then the plastic characteristics of such a metal will be much worse. Another requirement for hot rolled non-ferrous metals is extremely accurate temperature control of the metal.
For example, zinc requires a temperature of strictly 180 degrees when rolling - if it is slightly higher or slightly lower, the capricious metal will sharply lose its ductility.
Copper is more “loyal” to temperature (it can be rolled at 850 - 900 degrees), but it requires that the melting furnace must have an oxidizing (with a high oxygen content) atmosphere - otherwise it becomes brittle.
general characteristics
Metals are a group of elements, in the form of simple substances, that have metallic properties (ductility, malleability, luster, electronic conductivity, etc.)
The main difference between metal elements is that they have only reducing properties, and in reactions they can only oxidize. In compounds they can only have positive oxidation states, both in elementary positively charged ions and in complex ions, where they form positive centers.
Rice. 1. List of metals.
As a rule, at the outer level of metal elements there is a small number of electrons (1-3), electronegativity values are low. Metals include all s-elements (except hydrogen and helium), d- and f-elements, as well as p-elements under the boron-astatine line. Typical metals have large atomic sizes, which makes it easy for them to lose valence electrons. The resulting positive ions are stable because they have a complete outer electron shell.