Basic properties
Smelting copper from ore
Copper, as a metal, is obtained by smelting ore; in nature it is difficult to find pure nuggets; mainly enrichment and extraction is carried out from:
- chalcocite ore, in which the copper content is about 80%, this type is often called copper luster;
- Bronite ore, here the metal content is up to 65%
- covellite ore - up to 64%.
In terms of its physical properties, copper is a red-colored metal; a pink tint may be present in the section; it is classified as a heavy metal because it has a high density.
A distinctive characteristic is electrical conductivity. Due to this, the metal is widely used in the manufacture of cables and electrical wires. In this indicator, copper is second only to silver; in addition, there are a number of other physical characteristics:
- hardness - on the Brindel scale equals 35 kgf/mm²;
- elasticity - 132000 MN/m²;
- linear thermal expansion - 0.00000017 units;
- relative elongation - 60%;
- melting point - 1083 ºС;
- boiling point - 2600 ºС;
- thermal conductivity coefficient - 335 kcal/m*h*deg.
The main properties of copper include the elastic modulus, which is calculated by various methods:
The shear modulus is useful to know in the production of materials for the construction industry - it is a value that characterizes the degree of resistance to shear and deformation under the influence of various loads. The modulus calculated using Young's method shows how the metal will behave under uniaxial tension. The shear modulus characterizes the response of a metal to shear load. Poisson's ratio shows how a material behaves under uniform compression.
Development of mines for the extraction of copper and other metals
The chemical properties of copper describe the combination with other substances into alloys and possible reactions to an acidic environment. The most significant characteristic is oxidation. This process actively manifests itself during heating; already at a temperature of 375 ºC, copper oxide begins to form, or scale as it is called, which can affect the conductive functions of the metal and reduce them.
When copper reacts with a solution of an iron salt, it turns into a liquid state. This method is used to remove copper coating on various products.
Long stay in water causes cuprite
When copper is exposed to a humid environment for a long time, cuprite, a greenish coating, forms on its surface. This property of copper is taken into account when using metal to cover roofs. It is noteworthy that cuprite performs a protective function; the metal underneath does not deteriorate at all, even for a hundred years. The only opponents of copper roofs are environmentalists. They explain their position by the fact that when copper cuprite is washed off by rainwater into the soil or water bodies, it pollutes it with its toxins, which especially has a detrimental effect on microorganisms living in rivers and lakes. But to solve this problem, builders use drainpipes made of a special metal, which absorbs copper particles and accumulates them, while the water flows free of toxins.
Copper sulfate is another result of chemical action on metal. This substance is actively used by agronomists to fertilize the soil and stimulate the growth of various crops. However, uncontrolled use of vitriol can also have a detrimental effect on the environment. Toxins penetrate deep into the layers of the earth and accumulate in groundwater.
How do you find the value?
The density of metals is a characteristic that can be determined in two fundamentally different ways:
- experimental;
- theoretical.
Experimental methods are of the following types:
- Direct measurements of body weight and volume. The latter is easy to calculate if the geometric parameters of the body are known and its shape is ideal, for example, a prism, pyramid or ball.
- Hydrostatic measurements. In this case, special scales are used, invented by Galileo in the 16th century. The principle of their operation is quite simple: first, a body of unknown density is weighed in air, and then in liquid (water). After this, the required value is calculated using a simple formula.
As for the theoretical method of determining the density of metals, this is a fairly simple method that requires knowledge of the type of crystal lattice, the interatomic distance in it and the mass of the atom. Next, using the example of osmium, we will show how this method is used.
What is copper
One of the most common non-ferrous metals used in industry is copper, its Latin name is Cuprum, after the island of Cyprus, where it was mined by the Greeks many thousands of years ago. This is one of the seven metals that were known in ancient times; jewelry, dishes, money, and tools were made from it. Historians even called the period (from the 4th to the 3rd millennium BC) the Copper Age. D.I. Mendeleev put this metal in 29th place in his table, after hydrogen, since copper does not displace it from an acidic environment. Copper is a non-ferrous metal that has unique physical, mechanical, and chemical properties. The density of copper in kg m³ is one of the most important characteristics; it is used to determine the weight of the future product.
Density of metals
This is the most numerous group of the periodic table. A metal is any substance that has high thermal and electrical conductivity, a characteristic surface shine when polished, and the ability to undergo plastic deformation.
This chemical element has low electronegativity compared to substances such as nitrogen, oxygen and carbon. This fact leads to the fact that in bulk structures metal atoms form metallic bonds with each other. It represents the electrical interaction between positively charged ionic bases and a negative electron gas.
Metal atoms are arranged in space in an ordered structure called a crystal lattice. There are only three types:
- cubic;
- BCC (body-centered cubic);
- HPU (hexagonal close-packed);
- FCC (face centered cubic).
The density of metals is a physical quantity that depends on the type of crystal lattice. Below is a table of this parameter for all chemical elements in g/cm3, which under normal conditions are in a solid state.
It follows from the table that the density of metals is a value that varies over a wide range. Thus, the weakest is lithium, which, with the same volumes, is two times lighter than water. The density of the rare metal osmium is the highest in nature. It is 22.59 g/cm3.
Specific gravity of aluminum kg m3 – Telegraph
All metals have certain physical and mechanical properties, which, in fact, determine their specific gravity. To determine how suitable a particular alloy of ferrous or stainless steel is for production, the specific gravity of rolled metal is calculated.
All metal products that have the same volume, but are made from different metals, for example, iron, brass or aluminum, have different mass, which is directly dependent on its volume. In other words, the ratio of the volume of the alloy to its mass—specific density (kg/m3)—is a constant value that will be characteristic of a given substance.
The density of the alloy is calculated using a special formula and is directly related to the calculation of the specific gravity of the metal.
The specific gravity of a metal is the ratio of the weight of a homogeneous body of this substance to the volume of the metal, i.e. this is density, in reference books it is measured in kg/m3 or g/cm3. From here you can calculate the formula for finding out the weight of a metal. To find this you need to multiply the reference density value by the volume.
The table shows the densities of non-ferrous metals and ferrous iron.
The table is divided into groups of metals and alloys, where under each name the grade according to GOST and the corresponding density in g/cm3 are indicated, depending on the melting point.
To determine the physical value of specific density in kg/m3, you need to multiply the tabulated value in g/cm3 by 1000. For example, this way you can find out what the density of iron is - 7850 kg/m3.
The most typical ferrous metal is iron. The density value of 7.85 g/cm3 can be considered the specific gravity of iron-based ferrous metal.
Ferrous metals in the table include iron, manganese, titanium, nickel, chromium, vanadium, tungsten, molybdenum, and ferrous alloys based on them, for example, stainless steel (density 7.7-8.0 g/cm3), black steel ( density 7.85 g/cm3) cast iron (density 7.0-7.3 g/cm3) is mainly used. The remaining metals are considered non-ferrous, as well as alloys based on them. Non-ferrous metals in the table include the following types:
− light – magnesium, aluminum;
− noble metals (precious) – platinum, gold, silver and semi-precious copper;
− low-melting metals – zinc, tin, lead.
| Name of metal, designation | Atomic weight | Melting point, °C | Specific gravity, g/cc |
| Zinc Zn (Zinc) | 65,37 | 419,5 | 7,13 |
| Aluminum Al | 26,9815 | 659 | 2,69808 |
| Lead Pb (Lead) | 207,19 | 327,4 | 11,337 |
| Tin Sn (Tin) | 118,69 | 231,9 | 7,29 |
| Copper Cu (Copper) | 63,54 | 1083 | 8,96 |
| Titanium Ti (Titanium) | 47,90 | 1668 | 4,505 |
| Nickel Ni (Nickel) | 58,71 | 1455 | 8,91 |
| Magnesium Mg (Magnesium) | 24 | 650 | 1,74 |
| Vanadium V | 6 | 1900 | 6,11 |
| Tungsten W (Wolframium) | 184 | 3422 | 19,3 |
| Chrome Cr (Chromium) | 51,996 | 1765 | 7,19 |
| Molybdenum Mo (Molybdaenum) | 92 | 2622 | 10,22 |
| Silver Ag (Argentum) | 107,9 | 1000 | 10,5 |
| Tantalum Ta (Tantal) | 180 | 3269 | 16,65 |
| Iron Fe (Iron) | 55,85 | 1535 | 7,85 |
| Gold Au (Aurum) | 197 | 1095 | 19,32 |
| Platinum Pt (Platina) | 194,8 | 1760 | 21,45 |
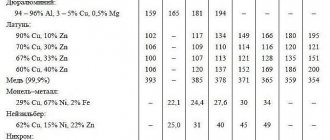
Table of specific gravity of metal alloys
The specific gravity of metals is most often determined in laboratory conditions, but in their pure form they are very rarely used in construction. Alloys of non-ferrous metals and alloys of ferrous metals, which according to their specific gravity are divided into light and heavy, are much more often used.
Light alloys are actively used by modern industry due to their high strength and good high-temperature mechanical properties. The main metals of such alloys are titanium, aluminum, magnesium and beryllium. But alloys based on magnesium and aluminum cannot be used in aggressive environments and at high temperatures.
Heavy alloys are based on copper, tin, zinc, and lead. Among the heavy alloys, bronze (an alloy of copper with aluminum, an alloy of copper with tin, manganese or iron) and brass (an alloy of zinc and copper) are used in many industries. Architectural parts and sanitary fittings are produced from these grades of alloys.
The reference table below shows the main quality characteristics and specific gravity of the most common metal alloys. The list provides data on the density of the main metal alloys at an ambient temperature of 20°C.
| List of metal alloys | Density of alloys (kg/m3) |
| Admiralty Brass – Admiralty Brass (30% zinc, and 1% tin) | 8525 |
| Aluminum bronze – Aluminum Bronze (3-10% aluminum) | 7700 – 8700 |
| Babbitt – Antifriction metal | 9130 -10600 |
| Beryllium bronze (beryllium copper) – Beryllium Copper | 8100 – 8250 |
| Delta metal – Delta metal | 8600 |
| Yellow brass – Yellow Brass | 8470 |
| Phosphorous bronzes – Bronze – phosphorous | 8780 – 8920 |
| Regular bronzes – Bronze (8-14% Sn) | 7400 – 8900 |
| Inconel | 8497 |
| Incoloy | 8027 |
| Wrought Iron | 7750 |
| Red brass (low zinc) – Red Brass | 8746 |
| Brass, casting – Brass – casting | 8400 – 8700 |
| Brass, rolled – Brass – rolled and drawn | 8430 – 8730 |
Steel classification
Depending on the proportion of non-metallic impurities determined by the method of smelting a given grade, steel alloys are divided into:
- especially high quality;
- high quality;
- ordinary quality.
Based on their chemical composition, alloys are also divided into alloyed and carbon.
Carbon steels
Used primarily for the production of welded structures and contains from 0.25 to 2.14 percent carbon. Within the group, they are further divided into subgroups, and also according to the percentage of carbon:
- high carbon (0.6-2.14);
- medium carbon (0.3-0.55);
- low carbon (below 0.25).
They also contain silicon and manganese as additives. In addition to useful, purposefully introduced additives, the alloy may also contain harmful impurities that negatively affect its physicochemical properties:
- phosphorus reduces ductility when heated and increases brittleness when cooled;
- sulfur leads to the formation of microcracks.
Other impurities may also be present in the alloy.
Alloy steel
In order for the alloy to acquire the required properties during melting, useful additives or alloying elements are added to it, most often metals such as aluminum, molybdenum, chromium, manganese, nickel, vanadium and others.
The properties of the alloy change quite significantly: the alloy acquires resistance to corrosion, special strength, high malleability, increased or decreased electrical conductivity, etc. An alloy with such additives is called alloy steel.
Based on the percentage of alloying additives, they are divided into three groups:
- highly alloyed – over 11;
- medium alloyed – from 4 to 11;
- low alloy – less than 4.
By area of application, steel alloys are divided into:
- tool - high-strength alloys are used for the manufacture of tools, dies, cutters, drills and cutters;
- structural - used for the production of bodies and components of vehicles, machine tools, building structures;
- special. This group includes alloys with increased resistance to acidic and alkaline environments, radiation, stainless alloys, electrical materials, etc.
Some additives and treatments increase the density of the material, while others reduce it, for example:
| Processing method or additive | Density change |
| carbon | is decreasing |
| chrome, aluminum, manganese | is decreasing |
| cobalt, tungsten, copper | growing |
| drawing | grows within three percent |
, please select a piece of text and press Ctrl+Enter.
To quickly search for a specific material or substance, press Ctrl+F.
This page presents a table of densities of basic materials (metals, rubbers, wood, gases, oils) under normal conditions.
| Material | Density, kg/m3 |
| Steel 10 GOST 1050-88 | 7856 |
| Steel 20 GOST 1050-88 | 7859 |
| Steel 40 GOST 1050-88 | 7850 |
| Steel 60 GOST 1050-88 | 7800 |
| S235-S375 GOST 27772-88 | 7850 |
| St3ps GOST 380-2005 | 7850 |
‘);> //–>
‘);> //–>
| Malleable cast iron KCH 70-2 GOST 1215-79 | 7000 |
| High-strength cast iron HF35 GOST 7293-85 | 7200 |
| Gray cast iron SCh10 GOST 1412-85 | 6800 |
| Gray cast iron SCH20 GOST 1412-85 | 7100 |
| Gray cast iron SCh30 GOST 1412-85 | 7300 |
| Silumin AK12zh GOST 1583-93 | 2700 |
| Alloy AK12 GOST 1583-93 | 2710 |
| Alloy AK5M GOST 1583-93 | 2640 |
| Alloy AK7 GOST 1583-93 | 2700 |
| Alloy AO9-1 GOST 14113-78 | 2700 |
| B83 GOST 1320-74 | 7380 |
| B87 GOST 1320-74 | 7300 |
| BN GOST 1320-74 | 9550 |
| Alloy ML10. ML19 GOST 2856-79 | 1810 |
| Alloy VML5 | 1890 |
| Alloy VML9 | 1850 |
| Tin bronze BrO10C10 | 8800 |
| Tin bronze BrO19 | 8600 |
| Tin bronze BrOS10-10 | 9100 |
| Tin bronze BrОA10-1 | 8750 |
| Bronze BrA10Zh3Mch2 GOST 493-79 | 8200 |
| Bronze BrA9ZH3L GOST 493-79 | 8200 |
| Bronze BrMts5 GOST 18175-78 | 8600 |
| Brass L60 GOST 15527-2004 | 8800 |
| Brass LA GOST 1020-97 | 8500 |
| Copper M0, M1, M2, M3 GOST 859-2001 | 8940 |
| Copper MSr1 GOST 16130-90 | 8900 |
| VT1-0 GOST 19807-91 | 4500 |
| VT14 GOST 19807-91 | 4500 |
| VT20L GOST 19807-91 | 4470 |
| F-4 GOST 10007-80 E | 2100 |
| Fluoroplastic – 1 GOST 13744-87 | 1400 |
| Fluoroplastic – 2 GOST 13744-87 | 1700 |
| Fluoroplastic – 3 GOST 13744-87 | 2710 |
| Fluoroplastic – 4D GOST 14906-77 | 2150 |
| Dakryl-2M TU 2216-265-057 57 593-2000 | 1190 |
| Polymethyl methacrylate LPT TU 6-05-952-74 | 1180 |
Areas of copper use
Due to its mechanical properties, copper has found wide application in various industries, but most often it can be found as an integral part of electrical wires, in heating and air cooling systems, in the production of computer equipment, and heat exchangers.
Industry uses thousands of tons of copper annually
In construction, this metal is used in the manufacture of various structures; the main advantage here is the low volumetric weight of copper. As noted above, non-ferrous metal has found wide application in roofing work, as well as in the manufacture of pipes. The resulting pipes are lightweight and can be transformed, which is especially important when designing water supply and sewerage systems.
The main part of the production of copper products is wire used as a core for electrical or communication cables. Due to the main characteristic of copper - electrical conductivity, it has a high resistance to current, and also has unique magnetic properties - unlike other metals, its particles do not react to a magnet, which sometimes complicates the process of cleaning it. It is worth noting that almost all production of products is based on the processing of secondary raw materials; ore is used extremely rarely.