Thermal conductivity.
So what is thermal conductivity? From a physics point of view, thermal conductivity
– this is the molecular transfer of heat between directly contacting bodies or particles of the same body with different temperatures, in which the energy of movement of structural particles (molecules, atoms, free electrons) is exchanged.
To put it simply, thermal conductivity
is the ability of a material to conduct heat. If there is a temperature difference inside the body, then thermal energy moves from the hotter part of the body to the colder part. Heat transfer occurs due to the transfer of energy when molecules of a substance collide. This happens until the temperature inside the body becomes the same. This process can occur in solid, liquid and gaseous substances.
In practice, for example in construction for the thermal insulation of buildings, another aspect of thermal conductivity is considered, associated with the transfer of thermal energy. Let's take "abstract house" as an example. In the “abstract house” there is a heater that maintains a constant temperature inside the house, say, 25 ° C. The temperature outside is also constant, for example, 0 °C. It is quite clear that if you turn off the heater, then after a while the house will also be 0 °C. All the heat (thermal energy) will go through the walls to the street.
To maintain the temperature in the house at 25 ° C, the heater must be constantly running. The heater constantly creates heat, which constantly escapes through the walls to the street.
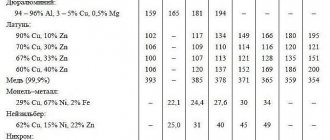
Impurities in copper alloys
from here
Impurities contained in copper (and, naturally, interacting with it) are divided into three groups.
Forming solid solutions with copper
Such impurities include aluminum, antimony, nickel, iron, tin, zinc, etc. These additives significantly reduce electrical and thermal conductivity. The grades that are primarily used for the production of conductive elements include M0 and M1. If the copper alloy contains antimony, its hot pressure treatment becomes significantly more difficult.
Impurities that do not dissolve in copper
These include lead, bismuth, etc. Although they do not affect the electrical conductivity of the base metal, such impurities make it difficult to process by pressure.
Impurities that form brittle chemical compounds with copper
This group includes sulfur and oxygen, which reduces the electrical conductivity and strength of the base metal. The presence of sulfur in the copper alloy greatly facilitates its machinability by cutting.
Coefficient of thermal conductivity.
The amount of heat that passes through the walls (and according to science, the intensity of heat transfer due to thermal conductivity) depends on the temperature difference (in the house and outside), on the area of the walls and the thermal conductivity of the material from which these walls are made.
To quantify thermal conductivity, there is a coefficient of thermal conductivity of materials . This coefficient reflects the property of a substance to conduct thermal energy. The higher the thermal conductivity coefficient of a material, the better it conducts heat. If we are going to insulate a house, then we need to choose materials with a small value of this coefficient. The smaller it is, the better. Nowadays, the most widely used materials for insulating buildings are mineral wool insulation and various foam plastics. A new material with improved thermal insulation properties – Neopor – is gaining popularity.
The thermal conductivity coefficient of materials is designated by the letter ? (Greek small letter lambda) and is expressed in W/(m2*K). This means that if we take a brick wall with a thermal conductivity coefficient of 0.67 W/(m2*K), a thickness of 1 meter and an area of 1 m2, then with a temperature difference of 1 degree, 0.67 watts of thermal energy will pass through the wall energy. If the temperature difference is 10 degrees, then 6.7 watts will pass. And if, with such a temperature difference, the wall is made 10 cm, then the heat loss will already be 67 watts. More details about the methodology for calculating heat loss in buildings can be found here.
It should be noted that the values of the thermal conductivity coefficient of materials are indicated for a material thickness of 1 meter. To determine the thermal conductivity of a material for any other thickness, the thermal conductivity coefficient must be divided by the desired thickness, expressed in meters.
In building codes and calculations the concept of “thermal resistance of a material” is often used. This is the reciprocal of thermal conductivity. If, for example, the thermal conductivity of foam plastic 10 cm thick is 0.37 W/(m2*K), then its thermal resistance will be equal to 1/0.37 W/(m2*K) = 2.7 (m2*K)/ Tue
Melting point of bronze
The melting point of bronze ranges from 854 to 1135°C. Bronze AZHN11-6-6 has the highest melting point - it melts at a temperature of 1408 K (1135°C). The melting point of this bronze is even higher than the melting point of copper, which is 1084.6°C.
Bronzes with a low melting point include: BrOTs8-4, BrB2, BrMTsS8-20, BrSN60-2.5 and the like.
Melting point of bronze
| Bronze | t, °С | Bronze | t, °С |
| BrA5 | 1056 | BrOS8-12 | 940 |
| BrA7 | 1040 | BrOSN10-2-3 | 1000 |
| BrA10 | 1040 | BrOF10-1 | 934 |
| BrAZH9-4 | 1040 | BrOF4-0.25 | 1060 |
| BrAZhMC10-3-1.5 | 1045 | BrOTs10-2 | 1015 |
| BrAZHN10-4-4 | 1084 | BrOTs4-3 | 1045 |
| BrAZHN11-6-6 | 1135 | BrOTs6-6-3 | 967 |
| BrAZhS7-1.5-1.5 | 1020 | BrOTs8-4 | 854 |
| BrAMTS9-2 | 1060 | BrOTsS3.5-6-5 | 980 |
| BrB2 | 864 | BrOTsS4-4-17 | 920 |
| BrB2.5 | 930 | BrOTsS4-4-2.5 | 887 |
| BrKMTs3-1 | 970 | BrOTsS5-5-5 | 955 |
| BrKN1-3 | 1050 | BrOTsS8-4-3 | 1015 |
| BrKS3-4 | 1020 | BrOTsS3-12-5 | 1000 |
| BrKTs4-4 | 1000 | BrOTsSN3-7-5-1 | 990 |
| BrMG0.3 | 1076 | BrS30 | 975 |
| BrMC5 | 1007 | BrSN60-2.5 | 885 |
| BrMTsS8-20 | 885 | BrSUN7-2 | 950 |
| BrO10 | 1020 | BrХ0.5 | 1073 |
| BrOS10-10 | 925 | BrTsr0.4 | 965 |
| BrOS10-5 | 980 | Cadmium | 1040 |
| BrOS12-7 | 930 | Silver | 1082 |
| BrOS5-25 | 899 | HOT alloy | 1075 |
Note: The melting and boiling points of other common metals are given in this table.
- Physical quantities. Directory. Ed. I.S. Grigorieva, E.Z. Meilikhova. - M.: Energoatomizdat, 1991. - 1232 p.
- Chirkin V.S. Thermophysical properties of nuclear technology materials. M.: Atomizdat, 1967 - 474 p.
Thermal conductivity coefficient of materials.
The table below shows the values of the thermal conductivity coefficient for some materials used in construction.
| Material | Coeff. warm W/(m2*K) |
| Alabaster slabs | 0,470 |
| Aluminum | 230,0 |
| Asbestos (slate) | 0,350 |
| Fibrous asbestos | 0,150 |
| Asbestos cement | 1,760 |
| Asbestos cement slabs | 0,350 |
| Asphalt | 0,720 |
| Asphalt in floors | 0,800 |
| Bakelite | 0,230 |
| Concrete on crushed stone | 1,300 |
| Concrete on sand | 0,700 |
| Porous concrete | 1,400 |
| Solid concrete | 1,750 |
| Thermal insulating concrete | 0,180 |
| Bitumen | 0,470 |
| Paper | 0,140 |
| Light mineral wool | 0,045 |
| Heavy mineral wool | 0,055 |
| Cotton wool | 0,055 |
| Vermiculite sheets | 0,100 |
| Woolen felt | 0,045 |
| Construction gypsum | 0,350 |
| Alumina | 2,330 |
| Gravel (filler) | 0,930 |
| Granite, basalt | 3,500 |
| Soil 10% water | 1,750 |
| Soil 20% water | 2,100 |
| Sandy soil | 1,160 |
| The soil is dry | 0,400 |
| Compacted soil | 1,050 |
| Tar | 0,300 |
| Wood - boards | 0,150 |
| Wood - plywood | 0,150 |
| Hardwood | 0,200 |
| Chipboard | 0,200 |
| Duralumin | 160,0 |
| Reinforced concrete | 1,700 |
| Wood ash | 0,150 |
| Limestone | 1,700 |
| Lime-sand mortar | 0,870 |
| Iporka (foamed resin) | 0,038 |
| Stone | 1,400 |
| Multilayer construction cardboard | 0,130 |
| Foamed rubber | 0,030 |
| Natural rubber | 0,042 |
| Fluorinated rubber | 0,055 |
| Expanded clay concrete | 0,200 |
| Silica brick | 0,150 |
| Hollow brick | 0,440 |
| Silicate brick | 0,810 |
| Solid brick | 0,670 |
| Slag brick | 0,580 |
| Siliceous slabs | 0,070 |
| Brass | 110,0 |
| Ice 0°C | 2,210 |
| Ice -20°С | 2,440 |
| Linden, birch, maple, oak (15% humidity) | 0,150 |
| Copper | 380,0 |
| Mipora | 0,085 |
| Sawdust - backfill | 0,095 |
| Dry sawdust | 0,065 |
| PVC | 0,190 |
| Foam concrete | 0,300 |
| Polystyrene foam PS-1 | 0,037 |
| Polyfoam PS-4 | 0,040 |
| Polystyrene foam PVC-1 | 0,050 |
| Foam resopen FRP | 0,045 |
| Expanded polystyrene PS-B | 0,040 |
| Expanded polystyrene PS-BS | 0,040 |
| Polyurethane foam sheets | 0,035 |
| Polyurethane foam panels | 0,025 |
| Lightweight foam glass | 0,060 |
| Heavy foam glass | 0,080 |
| Glassine | 0,170 |
| Perlite | 0,050 |
| Perlite-cement slabs | 0,080 |
| Sand 0% moisture | 0,330 |
| Sand 10% moisture | 0,970 |
| Sand 20% humidity | 1,330 |
| Burnt sandstone | 1,500 |
| Facing tiles | 1,050 |
| Thermal insulation tile PMTB-2 | 0,036 |
| Polystyrene | 0,082 |
| Foam rubber | 0,040 |
| Portland cement mortar | 0,470 |
| Cork board | 0,043 |
| Cork sheets are lightweight | 0,035 |
| Cork sheets are heavy | 0,050 |
| Rubber | 0,150 |
| Ruberoid | 0,170 |
| Slate | 2,100 |
| Snow | 1,500 |
| Scots pine, spruce, fir (450…550 kg/cub.m, 15% humidity) | 0,150 |
| Resinous pine (600…750 kg/cub.m, 15% humidity) | 0,230 |
| Steel | 52,0 |
| Glass | 1,150 |
| Glass wool | 0,050 |
| Fiberglass | 0,036 |
| Fiberglass | 0,300 |
| Wood shavings - stuffing | 0,120 |
| Teflon | 0,250 |
| Paper roofing felt | 0,230 |
| Cement boards | 1,920 |
| Cement-sand mortar | 1,200 |
| Cast iron | 56,0 |
| Granulated slag | 0,150 |
| Boiler slag | 0,290 |
| Cinder concrete | 0,600 |
| Dry plaster | 0,210 |
| Cement plaster | 0,900 |
| Ebonite | 0,160 |
Effect of carbon concentration
The carbon concentration in steel affects the amount of heat transfer:
- Low carbon steels have a high conductivity index. That is why they are used in the manufacture of pipes, which are then used to create the heating system pipeline. The coefficient value varies from 54 to 47 W/(m* K).
- The average coefficient for common carbon steels is a value from 50 to 90 W/(m* K). That is why such material is used in the manufacture of parts for various mechanisms.
- For metals that do not contain various impurities, the coefficient is 64 W/(m* K). This value does not change significantly under thermal influence.
Thus, the considered indicator for alloyed alloys may vary depending on the operating temperature.
Application area
Ceramics made on the basis of aluminum nitride ALN have a fairly wide range of applications and will allow you to effectively solve problems, regardless of the level of complexity:
– blank for ceramic printed circuit boards requiring high reliability indicators;
– production of substrates for semiconductors; – as a heat-absorbing element in LED circuits and high-power electronic devices; – base for high-frequency resistors and as housings for various circuits; – substrate for laser diodes, semiconductor crystals; – for the manufacture of sensors and other devices operated in the most difficult conditions, etc.
from here
Characteristics
| Properties | Material | |||
| AlN-170 | AlN-200 | AlN-230 | ||
| Color | grey | grey | grey | |
| Bulk Density | g/cm3 | 3,30 | 3,28 | 3,25 |
| Ground surface roughness (Ra) | µm | 0,3-0,5 | 0,3-0,5 | 0,3-0,5 |
| Polished surface roughness (Ra) | µm | |||
| Mechanical characteristics | ||||
| Flexural strength | MPa | 450 | 250 | 200 |
| Elastic modulus | GPa | 320 | 320 | − |
| Hardness | kg/mm 2 | 11 | 11 | − |
| physical characteristics | ||||
| Coefficient of thermal expansion (40-800°C) | 10 -6 /°C | 5,4 | 5,4 | 9,0 |
| Thermal conductivity (25°C) | W/m∙°K | 180 | 200 | 230 |
| Specific heat | J/Kg∙°K | 720 | 720 | 750 |
| Dielectric constant (1 MHz) | – | 9,0 | 9,0 | 9,8 |
| Dielectric loss (1MHz, 25°C) | 10 -4 | 3 | 3 | 3 |
| Technological characteristics | ||||
| DBC technology | ||||
| (Cu 127 – 450 µm, protective coatings) | ||||
| Thick film technology | ||||
| (Ag, Au, Ag-Pd, Ag-Pd-Pt, Ni – 12-100 microns) | ||||
| Thin film technology | ||||
| (guides on request) | ||||
| Distance between scribing lines, mm | 2,00 ± 0,05 | |||
| Minimum hole diameter, mm | 0,20 ± 0,05 | |||