What is copper?
Copper is a ductile, but at the same time durable metal of a reddish-brown color. It is one of the main elements of the periodic table. The copper-based alloy is used to make other metals such as bronze and brass.
The scope of application of this metal is extremely wide. Due to its strength, it is in great demand in construction for the manufacture of wires, fittings, copper mesh, wires and other things. In the agricultural industry, copper is used as part of copper sulfate. Most wind musical instruments are made from copper. This metal is also widely used in the creation of plumbing pipes.
Despite the fact that copper is not a precious metal, it is also in demand in jewelry. You can find a lot of beautiful and very elegant copper jewelry: bracelets, crosses, chains, earrings, rings, etc. Also, to make gold jewelry more durable, an admixture of copper is used.
Products made from this metal can often be found in the kitchen. Due to its resistance to corrosion and good heat conductivity, copper is used to make a huge variety of kitchen utensils: , etc.
It would also not be superfluous to add that in the old days, along with gold and silver, copper was used to make coins, which the common people called copper coins . Many coins have survived to this day.
Unfortunately, in the absence of proper and timely care, copper objects can lose their original shine, become dirty and become cloudy. Let's talk about the main rules that must be followed when caring for copper products.
Reasons for oxidation of jewelry
The oxidation mechanism involves the introduction of oxygen molecules into the surface structure of the metal. Each metal reacts to this in its own way: it darkens, becomes covered with rust or patina.
Pure gold, silver and platinum group metals do not oxidize. But they are not used in their pure form to make jewelry: other metals - alloys - are added to them. The more alloys, the faster the jewelry alloys oxidize.
Jewelry alloys oxidize much more intensely than jewelry alloys. Metals containing iron are characterized by the appearance of a loose red layer - rust. But alloys that contain copper react differently to the penetration of oxygen: they darken and become covered with a bluish-green coating. This is a noble patina that can be seen on antique products made from copper and alloys containing it. Sometimes modern copper products (such as door handles or staircase elements) are deliberately patinated, giving them an antique appearance.
Jewelry specially coated with patina can be found on the websites of craftsmen. They are practically not produced commercially. As a rule, oxidation of jewelry is considered an unfavorable phenomenon that spoils its aesthetic appearance.
If jewelry oxidizes, you should try to bring it back to life or simply throw it away. Nothing spoils an outfit more than peeling or darkened jewelry!
Almost all jewelry alloys contain copper. This applies to both red and yellow (brass, bronze, tombac, randol) and white (nickel silver, nickel silver, alpaca and others like them) alloys. Therefore, the main “disease” of jewelry alloys is greening.
Silver earrings with ceramics and cubic zirconia (go to the SUNLIGHT catalogue)
Another metal that is added to most alloys is zinc. This metal, for example, is part of tombac, which is very similar to gold, which is often used by scammers. The zinc alloy in costume jewelry darkens, forming an unaesthetic coating, so that counterfeit jewelry inevitably reveals its ignoble origin over time.
High-quality jewelry is produced mainly from metals with a durable coating. The base is usually brass or another metal, and gold or silver is used as a coating. The thicker the precious coating, the better and more expensive the product.
There are a number of factors that help accelerate oxidation processes. This impact:
- high humidity and water (especially salty);
- temperature changes and heat;
- chemicals used in everyday life;
- cosmetics, hygiene products, perfume;
- natural body secretions.
It is impossible to completely protect your beloved jewelry from all these factors, but it is entirely within your power to minimize their harmful effects.
How to clean copper coins yourself
Source: Unsplash (@kdghantous)
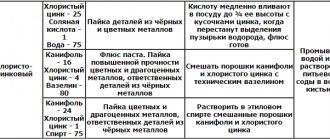
If you have copper coins in your hands, then congratulations, you are the owner of antique value. You need to treat such a treasure with care, because you can accidentally remove important elements. There are several ways to clean carefully. You need to select them depending on what kind of plaque has formed on the surface of the coin.
- If the coating is yellowish, it means the coin contains more lead than copper. You need to clean off such deposits with 9% vinegar. Add it to a small container of water and put the coins in it. When the dirt is gone, the mixture must be carefully drained and the coins rinsed in warm water. Important! Until they are washed, you cannot handle them with unprotected hands!
- Green plaque can be better cleaned off with 10% citric acid diluted in a glass of water in a ratio of 1:10. Place coins in the solution and watch the reaction. As soon as the liquid turns green, remove the money. The final steps are washing and light polishing.
- The reddish oxide will come off if you dip the coin in a solution of ammonia or ammonium carbonate. You need to keep coins in this solution for several hours. Otherwise it will not be cleansed.
- Black deposits are removed by ammonia. In this case, it will also take several hours of soaking to see the result.
Regardless of the method you choose, do not forget about safety. Use gloves and a respirator. Important! Copper coins must not be cleaned with abrasives, potent drugs, concentrated acids and alkalis. Do not try to remove dirt by exposing it to high temperatures. This may cause the product to melt or lose its strength.
What to do if the jewelry has already oxidized?
What to do if the jewelry darkens, despite all your tricks? You can wash it in a soapy solution, clean it with some food acid (for example, lemon juice or vinegar) or a solution of ammonia. If we are talking about uncoated jewelry, light abrasives such as tooth powder, soda slurry, or GOI paste are quite appropriate. You can also restore oxidized jewelry in a workshop. There, jewelry is cleaned with ultrasound in special cavitation baths. However, it must be taken into account that for this purpose the product sometimes has to be disassembled, which significantly affects the price of the service. So it’s better to store and wear your favorite jewelry correctly than to later pay money to have it professionally cleaned!
24.11.21