Density and other properties of carbon dioxide CO2 depending on temperature and pressure
The table shows the thermophysical properties of carbon dioxide CO2 depending on temperature and pressure.
The properties in the table are indicated at temperatures from 273 to 1273 K and pressures from 1 to 100 atm. Let's consider such an important property of carbon dioxide as density. The density of carbon dioxide is 1.913 kg/m3 under normal conditions (at N.S.). According to the table, it can be seen that the density of carbon dioxide significantly depends on temperature and pressure - with increasing pressure, the density of CO2 increases significantly, and with increasing gas temperature, it decreases. Thus, when heated by 1000 degrees, the density of carbon dioxide decreases by 4.7 times.
However, as the pressure of carbon dioxide increases, its density begins to increase, much more than it decreases when heated. For example, at a pressure of 10 atm. and a temperature of 0°C, the density of carbon dioxide already increases to a value of 20.46 kg/m3.
It should be noted that an increase in gas pressure leads to a proportional increase in the value of its density, that is, at 10 atm. The specific gravity of carbon dioxide is 10 times greater than at normal atmospheric pressure.
The table shows the following thermophysical properties of carbon dioxide:
- carbon dioxide density in kg/m3;
- specific heat capacity, kJ/(kg deg);
- thermal conductivity, W/(m deg);
- dynamic viscosity, Pa s;
- thermal diffusivity, m2/s;
- kinematic viscosity, m2/s;
- Prandtl number.
Note: Be careful! Thermal conductivity in the table is indicated to the power of 102. Don't forget to divide by 100!
Carbon dioxide. Physical properties. Gas mixtures.
Carbon dioxide.
GOST
8050
-
85
Carbon dioxide, gaseous and liquid
https://docs.cntd.ru/...ment/1200005325
Physical properties of carbon dioxide
Carbon dioxide (CO2, carbon dioxide, carbon dioxide) is a substance with the chemical formula CO2 and a molecular weight of 44.011 g/mol, which can exist in four phase states - gaseous, liquid, solid and supercritical. The gaseous state of CO2 is commonly called carbon dioxide. At atmospheric pressure it is a colorless, odorless gas, at a temperature of +20? With a density of 1.839 kg/m? (1.52 times heavier than air), dissolves well in water (0.88 volumes in 1 volume of water), partially interacting in it with the formation of carbonic acid. Included in the atmosphere is an average of 0.035% by volume. During sudden cooling due to expansion (expansion), CO2 is able to desublimate - go directly into the solid state, bypassing the liquid phase.
The liquid state of CO2 is technically called “liquid carbon dioxide” or simply “carbon dioxide”. This is a colorless, odorless liquid with an average density of 771 kg/m3, which exists only under a pressure of 3,482...519 kPa at a temperature of 0...-56.5 degrees C (“low-temperature carbon dioxide”), or under a pressure of 3,482...7,383 kPa at a temperature of 0...+31.0 degrees C (“high pressure carbon dioxide”). High-pressure carbon dioxide is most often produced by compressing carbon dioxide to condensation pressure while simultaneously cooling with water. Low-temperature carbon dioxide, which is the main form of carbon dioxide for industrial consumption, is most often produced through a high-pressure cycle by three-stage cooling and throttling in special installations.
For low and medium consumption of carbon dioxide (high pressure), a variety of steel cylinders are used for its storage and transportation (from cylinders for household siphons to containers with a capacity of 55 liters). The most common is a 40 liter cylinder with an operating pressure of 15,000 kPa, containing 24 kg of carbon dioxide.
With an instantaneous decrease in pressure to atmospheric pressure, which occurs during injection into a special expansion chamber (throttling), liquid carbon dioxide instantly turns into gas and a thin snow-like mass, which is pressed and carbon dioxide is obtained in a solid state, which is commonly called “dry ice”. At atmospheric pressure, it is a white glassy mass with a density of 1,562 kg/m?, with a temperature of -78.5? C, which sublimates in the open air - gradually evaporates, bypassing the liquid state.
At a pressure above 7.39 kPa and a temperature above 31.6 degrees C, carbon dioxide is in the so-called supercritical state, in which its density is like that of a liquid, and its viscosity and surface tension are like those of a gas. This unusual physical substance (fluid) is an excellent non-polar solvent.
Specific gravity. The specific gravity of carbon dioxide depends on the pressure, temperature and state of aggregation in which it is located.
The critical temperature of carbon dioxide is +31 degrees. Specific gravity of carbon dioxide at 0 degrees and a pressure of 760 mm Hg. equal to 1.9769 kg/m3. The molecular weight of carbon dioxide is 44.0. The relative weight of carbon dioxide compared to air is 1.529. Liquid carbon dioxide at temperatures above 0 degrees. much lighter than water and can only be stored under pressure.
Triple point of carbon dioxide. The triple point is characterized by a pressure of 5.28 ata (kg/cm2) and a temperature of minus 56.6 degrees.
Carbon dioxide can exist in all three states (solid, liquid and gas) only at the triple point. At pressures below 5.28 ata (kg/cm2) (or at temperatures below minus 56.6 degrees), carbon dioxide can only exist in solid and gaseous states.
https://popgun.ru/vi...ic.php?t=469187
GOST R ISO 14175-2010 Welding materials. Gases and gas mixtures for fusion welding and related processes
Terms and Definitions
3.1 main gas
(base gas): The gas that makes up the majority of the volume of a gas mixture, or the only component of a pure gas.
3.3 component
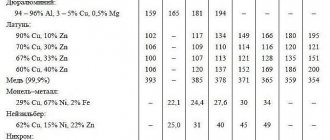
(component): A component of a gas mixture that affects the performance properties and characteristics of the gas mixture (for example, in a mixture containing 11% CO in argon, CO is considered a component, and argon is considered the main gas).
3.6 admixture
(impurity): A substance with a chemical composition different from the main gas and/or components.
3.7 gas mixture
(mixture): A gas consisting of a base gas and one or more components.
Example 4 - Gas mixture containing Ar as the main gas, 0.05% O as a component.
Classification: ISO 14175 - Z.https://docs.cntd.ru/...ment/1200084975
—-
The main gas and components in cylinders for welding mixtures are in a gaseous state.
In carbon dioxide cylinders, gas is in a liquid state.
The siphon tube in the cylinder is used to select the liquid phase. The siphon tube in cylinders for gas mixtures is used to mix the main gas and components when the cylinders are separately filled with these gases.
——
ABC
|
Gas
mixtures and mixed
gaseschrome-extension://mhjfbmdgcfjbbpaeojofohoefgiehjai/index.html
ъ
Thermophysical properties of carbon dioxide CO2 at atmospheric pressure
The table shows the thermophysical properties of carbon dioxide CO2 depending on temperature (in the range from -75 to 1500°C) at atmospheric pressure. The following thermophysical properties of carbon dioxide are given:
- dynamic viscosity, Pa s;
- thermal conductivity coefficient, W/(m deg);
- Prandtl number.
The table shows that with increasing temperature, the thermal conductivity and dynamic viscosity of carbon dioxide also increase. Note: Be careful! Thermal conductivity in the table is indicated to the power of 102. Don't forget to divide by 100!
Carbon dioxide and us: why CO2 is dangerous
Carbon dioxide is one of the metabolic products in the human body. It plays a large role in controlling breathing and blood supply to organs. An increase in CO2 levels in the blood causes blood vessels to dilate, thus being able to transport more oxygen to tissues and organs. Likewise, the respiratory system is forced to become more active if the concentration of carbon dioxide in the body increases. This property is used in ventilators to stimulate the patient’s own respiratory organs to greater activity.
In addition to the benefits mentioned above, excess CO2 concentrations can also cause harm to the body. Increased levels in the inhaled air lead to nausea, headache, suffocation and even loss of consciousness. The body protests against carbon dioxide and sends signals to the person. With a further increase in concentration, oxygen starvation, or hypoxia, develops. Co2 prevents oxygen from joining hemoglobin molecules, which move bound gases through the circulatory system. Oxygen starvation leads to decreased performance, weakened reactions and abilities to analyze the situation and make decisions, apathy and can lead to death.
Common symptoms of carbon dioxide poisoning
Such concentrations of carbon dioxide, unfortunately, are achievable not only in cramped mines, but also in poorly ventilated school classrooms, concert halls, office spaces and vehicles - anywhere where a large number of people accumulate in a confined space without sufficient air exchange with the environment.
Thermal conductivity of carbon dioxide CO2 depending on temperature and pressure
The table shows the thermal conductivity of carbon dioxide CO2 in the temperature range from 220 to 1400 K and at pressure from 1 to 600 atm. The data above in the table applies to liquid CO2.
It should be noted that the thermal conductivity of liquefied carbon dioxide decreases with increasing temperature , and increases with increasing pressure. Carbon dioxide (in the gas phase) becomes more thermally conductive, both with increasing temperature and with increasing pressure.
Thermal conductivity in the table is given in the dimension W/(m deg). Be careful! Thermal conductivity in the table is indicated to the power of 103. Don't forget to divide by 1000!
Thermal conductivity of carbon dioxide CO2 in the critical region
The table shows the thermal conductivity values of carbon dioxide CO2 in the critical region in the temperature range from 30 to 50°C and at pressure from 62 to 80 atm. Note: Be careful! Thermal conductivity in the table is indicated to the power of 103. Don't forget to divide by 1000! Thermal conductivity in the table is indicated in W/(m deg).
Thermal conductivity of dissociated carbon dioxide CO2 at high temperatures
The table shows the thermal conductivity values of dissociated carbon dioxide CO2 in the temperature range from 1600 to 4000 K and at pressure from 0.01 to 100 atm. Be careful! Thermal conductivity in the table is indicated to the power of 103. Don't forget to divide by 1000! Thermal conductivity in the table is indicated in W/(m deg).
Thermal conductivity of liquid carbon dioxide CO2
The table shows the thermal conductivity values of liquid carbon dioxide CO2 on the saturation line depending on temperature. Note: Be careful! Thermal conductivity in the table is indicated to the power of 103. Don't forget to divide by 1000! Thermal conductivity in the table is indicated in W/(m deg).
Sources: 1. Vargaftik N.B. Handbook on the thermophysical properties of gases and liquids. 2. Chirkin V.S. Thermophysical properties of nuclear technology materials. M.: Atomizdat, 1967. - 474 p.
Producing carbon dioxide:
In industry, carbon dioxide is formed in flue gases during the combustion of various organic and inorganic substances or as a by-product of chemical processes, for example, during the decomposition of natural carbonates (dolomite, limestone). Carbon dioxide is also produced as a by-product in air separation plants to produce pure oxygen, nitrogen and argon.
In laboratory conditions, carbon dioxide is obtained, for example, as a result of the following chemical reactions:
1. interaction between calcium carbonate and nitric acid:
CaCO3 + 2HNO3 → Ca(NO3)2 + CO2 + H2O,
2. as a result of the interaction of calcium carbonate with other mineral acids,
3. interaction of baking soda with citric acid or sour lemon juice,
4. carbon combustion reactions:
C + O2 → CO2.