Table of physical properties of aluminum
| Melting point Tmelt, °C | 660 |
| Boiling point Bp, °C | 2 327 |
| Latent heat of fusion, J/g | 393,6 |
| Thermal conductivity l, W/m •deg (at 20° C) | 228 |
| Heat capacity Ср, J/(g deg) (at 0–100°С) | 0,88 |
| Linear expansion coefficient α ×10-6, 1/°С (pro°С) | 24,3 |
| Electrical resistivity ρ ×10-8, Ohm× m (at 20°C) | 2,7 |
| Tensile strength σ in, MPa | 40–60 |
| Relative elongation δ, % | 40–50 |
| Brinell hardness HB | 25 |
| Modulus of normal elasticity E, GPa | 70 |
Thermal conductivity of steel and other alloys: copper, brass and aluminum, heat transfer
Before working with various metals and alloys, you should study all the information regarding their basic characteristics. Steel is the most common metal and is used in various industries. An important indicator is thermal conductivity, which varies over a wide range and depends on the chemical composition of the material and many other indicators.
This term means the ability of various materials to exchange energy , which in this case is represented by heat. In this case, energy transfer passes from the hotter part to the colder part and occurs due to:
- Molecules
- Atoms.
- Electrons and other particles of the metal structure.
The thermal conductivity of stainless steel will differ significantly from that of another metal - for example, the thermal conductivity of copper will be different than that of steel.
To indicate this indicator, a special value is used, called the thermal conductivity coefficient. It is characterized by the amount of heat that can pass through a material in a certain unit of time.
Indicators for steel
Thermal conductivity can vary significantly depending on the chemical composition of the metal. The coefficient of this value will be different for steel and copper. In addition, with an increase or decrease in carbon concentration, the indicator under consideration also changes.
There are other features of thermal conductivity:
- For steel that does not have impurities, the value is 70 W/(m* K).
- Carbon and high-alloy steels have much lower conductivity. Due to an increase in the concentration of impurities, it is significantly reduced.
- The thermal effect itself can also affect the structure of the metal. As a rule, after heating, the structure changes its conductivity value, which is associated with a change in the crystal lattice.
The thermal conductivity coefficient of aluminum is much higher, which is due to the lower density of this material. The thermal conductivity of brass also differs from that of steel.
Effect of carbon concentration
The carbon concentration in steel affects the amount of heat transfer:
- Low carbon steels have a high conductivity index. That is why they are used in the manufacture of pipes, which are then used to create the heating system pipeline. The coefficient value varies from 54 to 47 W/(m* K).
- The average coefficient for common carbon steels is a value from 50 to 90 W/(m* K). That is why such material is used in the manufacture of parts for various mechanisms.
- For metals that do not contain various impurities, the coefficient is 64 W/(m* K). This value does not change significantly under thermal influence.
Thus, the considered indicator for alloyed alloys may vary depending on the operating temperature.
Importance in everyday life and production
Why is it important to consider thermal conductivity? A similar value is indicated in various tables for each metal and is taken into account in the following cases:
- In the manufacture of various heat exchangers. Heat is one of the important carriers of energy. It is used to provide comfortable living conditions in residential and other premises. When creating heating radiators and boilers, it is important to ensure rapid and complete heat transfer from the coolant to the end consumer.
- In the manufacture of outlet elements. You can often encounter a situation where you need to remove heat rather than supply it. An example is the case of heat removal from the cutting edge of a tool or gear teeth. To ensure that the metal does not lose its basic performance qualities, rapid removal of thermal energy is ensured.
- When creating insulating layers. In some cases, the material should not conduct thermal energy transfer. For such operating conditions, a metal is selected that has a low heat conductivity coefficient.
The indicator under consideration is determined when testing under various conditions. As previously noted, the thermal conductivity coefficient may depend on the operating temperature. Therefore, the tables indicate several of its values.
Source: https://tokar.guru/metally/stal/teploprovodnost-stali-alyuminiya-latuni-medi.html
| Aluminum is a chemical element of the third group of the periodic table of D.I. Mendeleev. Table of physical properties of aluminum | |
| Melting point Tmelt, °C | 660 |
| Boiling point Bp, °C | 2 327 |
| Latent heat of fusion, J/g | 393,6 |
| Thermal conductivity l, W/m •deg (at 20° C) | 228 |
| Heat capacity Ср, J/(g deg) (at 0–100°С) | 0,88 |
| Linear expansion coefficient α ×10-6, 1/°С (pro°С) | 24,3 |
| Electrical resistivity ρ ×10-8, Ohm× m (at 20°C) | 2,7 |
| Tensile strength σ in, MPa | 40–60 |
| Relative elongation δ, % | 40–50 |
| Brinell hardness HB | 25 |
| Modulus of normal elasticity E, GPa | 70 |
Aluminum Density
The density of solid and molten aluminum decreases as its purity increases:
Density of aluminum at 20°C
| Purity degree, % | 99,25 | 99,40 | 99,75 | 99.97 | 99,996 | 99.9998 |
| Density at 20°C, g/cm3 | 2,727 | 2,706 | 2,703 | 2,6996 | 2,6989 | 2,69808 |
Density of molten aluminum at 1000°C
| Purity degree, % | 99,25 | 99.40 | 99.75 |
| Density, g/cm3 | 2,311 | 2,291 | 2,289 |
Melting and boiling points
At the moment of melting of aluminum, the volume of the metal increases: for aluminum with a purity of 99.65% - by 6.25%, for a purer metal - by 6.60%. As the purity of aluminum increases, its melting point increases:
Dependence of the melting temperature of aluminum on purity
| Purity degree, % | 99,2 | 99,5 | 99,6 | 99,97 | 99,996 |
| Melting point, °C | 657 | 658 | 659,7 | 659,8 | 660,24 |
Thermal conductivity of aluminum
The thermal conductivity of aluminum increases with increasing degree of its purity. For technical aluminum (99.49 and 99.70%), the thermal conductivity at 200°C is 209 and 222 W/(m×K), respectively. For electrolytically refined aluminum with a purity of 99.9%, the thermal conductivity at 190°C increases to 343 W/(m×K). Impurities of copper, magnesium and manganese in aluminum reduce its thermal conductivity. For example, the addition of 2% Mn to aluminum reduces thermal conductivity from 209 to 126 W/(m×K).
Electrical conductivity of aluminum
Aluminum has high electrical conductivity (fourth place among metals - after silver, copper and gold). The specific electrical conductivity of aluminum with a purity of 99.99% at 20°C is 37.9 μS×m, which is 63.7% of the electrical conductivity of copper [59.5 μS×m]. The purer aluminum [99.999%] has an electrical conductivity equal to 65.9% of copper. The electrical conductivity of aluminum is influenced by a number of factors: degree of deformation, heat treatment mode, etc.
, the decisive role is played by the nature of the impurities present in aluminum. Impurities, according to their negative effect on the electrical conductivity of aluminum, can be arranged in the following series: Cr, V, Mn, Ti, Mg, Ag, Cu, Zn, Si, Fe Ni.
The impurities of Cr, V, Mn and Ti have the most negative effect on the electrical resistance of aluminum.
Therefore, in aluminum for the electrical industry, the sum of Cr+V+Mn+Ti should not exceed 0.015% (grade A5E) and even 0.01% (A7E) with a silicon content of 0.12 and 0.16%, respectively.
The influence of impurities on the electrical conductivity of aluminum
The main impurities in aluminum are silicon, iron, copper, zinc and titanium. At low silicon contents in aluminum (0.06%), the Fe:Si value (ranging from 0.8 to 3.8) has a relatively small effect on its electrical resistance. As the silicon content increases to 0.15–0.16%, the influence of Fe:Si increases. Below is the effect of Fe:Si on the electrical conductivity of aluminum:
Effect of Fe:Si on the electrical conductivity of aluminum
| Fe:Si | 1,07 | 1,44 | 2,00 | 2,68 | 3,56 |
| Electrical resistivity of aluminum, ×10-2 μOhm mm: | |||||
| hard-worked | 2,812 | 2,816 | 2,822 | 2,829 | 2,838 |
| annealed | 2,769 | 2,771 | 2,778 | 2,783 | 2,788 |
The electrical resistivity of annealed aluminum wire (ρ, μΩ m) at 20°C, depending on the impurity content, can be approximately determined by the following formula: ρ=0.0264+0.007×(% Si)+0.0007×(% Fe) + 0.04×[% (Cr+V + Mn + Ti)].
Reflectivity
As the purity of aluminum increases, its ability to reflect light from a surface increases. Thus, the degree of reflection of white light from rolled aluminum sheets (foil), depending on the purity of the metal, increases as follows: for Al 99.2% - 75%, Al 99.5% - 84% and for Al 99.8% - 86 %. The surface of the sheet, made of electrolytically refined aluminum with a purity of 99.996%, reflects 90% of the white light incident on it.
Specific heat
Heat capacity is the amount of heat absorbed by a body when heated by 1 degree.
The heat capacity of a body is denoted by the capital Latin letter C.
What does the heat capacity of a body depend on? First of all, from its mass. It is clear that heating, for example, 1 kilogram of water will require more heat than heating 200 grams.
What about the type of substance? Let's do an experiment. Let's take two identical vessels and, having poured water weighing 400 g into one of them, and vegetable oil weighing 400 g into the other, we will begin to heat them using identical burners. By observing the thermometer readings, we will see that the oil heats up faster. To heat water and oil to the same temperature, the water must be heated longer. But the longer we heat the water, the more heat it receives from the burner.
Thus, different amounts of heat are required to heat the same mass of different substances to the same temperature. The amount of heat required to heat a body and, therefore, its heat capacity depend on the type of substance of which the body is composed.
So, for example, to increase the temperature of water weighing 1 kg by 1 °C, an amount of heat equal to 4200 J is required, and to heat the same mass of sunflower oil by 1 °C, an amount of heat equal to 1700 J is required.
A physical quantity showing how much heat is required to heat 1 kg of a substance by 1 °C is called the specific heat capacity of this substance.
Each substance has its own specific heat capacity, which is denoted by the Latin letter c and measured in joules per kilogram degree (J/(kg K)).
The specific heat capacity of the same substance in different states of aggregation (solid, liquid and gaseous) is different. For example, the specific heat capacity of water is 4200 J/(kg·K), and the specific heat capacity of ice is J/(kg·K); aluminum in the solid state has a specific heat capacity of 920 J/(kg·K), and in the liquid state - J/(kg·K).
Note that water has a very high specific heat capacity. Therefore, water in the seas and oceans, heating up in summer, absorbs a large amount of heat from the air. Thanks to this, in those places that are located near large bodies of water, summer is not as hot as in places far from the water.
Linear expansion coefficient of aluminum
- 1 Properties of aluminum: density, thermal conductivity, heat capacity Al 1.1 Specific heat capacity of aluminum
- 1.2 Thermophysical properties of aluminum alloys AMts, AMg, D16, AK, etc.
- 1.3 Thermal conductivity of aluminum alloys
- 1.4 Properties of aluminum alloys with silicon, copper, magnesium and zinc
- 1.5 Thermal conductivity of aluminum alloys depending on temperature
- 1.6 Thermal conductivity of aluminum alloy with lithium
- 1.7 Density, thermal conductivity, heat capacity of aluminum alloys Amts, Amg1, Amg2, D1, D16
- 1.8 Thermal conductivity, heat capacity and resistivity of alloy 1151T
- 1.9 Temperature coefficients of linear expansion (CTE) of alloy 1151T
- 1.10 Thermophysical properties of aluminum alloys of the Al-Cu-Mn system
- 1.11 Thermophysical properties of aluminum alloys of the Al-Mg-Si system
- 1.12 Specific heat capacity of high-strength aluminum alloys V93, alloy 1933, V95, alloy 1973, V96, etc.
- 1.13 Thermal conductivity of high-strength aluminum alloys V93, alloy 1933, V95, alloy 1973, V96, etc.
The table shows the thermophysical properties of aluminum Al depending on temperature. The properties of aluminum are given over a wide temperature range - from minus 223 to 1527 ° C (from 50 to 1800 K).
As can be seen from the table, the thermal conductivity of aluminum at room temperature is about 236 W/(m deg) , which makes it possible to use this material for the manufacture of radiators and various heat sinks.
In addition to aluminum, copper also has high thermal conductivity.
Which metal has the greater thermal conductivity? It is known that the thermal conductivity of aluminum at medium and high temperatures is still less than that of copper, however, when cooled to 50K, the thermal conductivity of aluminum increases significantly and reaches a value of 1350 W/(m deg). For copper, at such a low temperature, the thermal conductivity value becomes lower than for aluminum and amounts to 1250 W/(m deg).
Aluminum begins to melt at a temperature of 933.61 K (about 660 ° C), while some of its properties undergo significant changes. The values of properties such as thermal diffusivity, aluminum density and thermal conductivity are significantly reduced.
The density of aluminum is mainly determined by its temperature and depends on the state of aggregation of this metal.
For example, at a temperature of 27°C, the density of aluminum is 2697 kg/m3 , and when this metal is heated to the melting point (660°C), its density becomes equal to 2368 kg/m3.
The decrease in aluminum density with increasing temperature is due to its expansion when heated.
The table shows the following thermophysical properties of aluminum:
- aluminum density, g/cm3;
- specific (mass) heat capacity, J/(kg deg);
- thermal diffusivity coefficient, m2/s;
- thermal conductivity of aluminum, W/(m deg);
- electrical resistivity, Ohm m;
- Lorentz function.
Specific heat capacity of aluminum
The specific heat capacity of aluminum depends significantly on temperature and at room temperature is about 904 J/(kg deg) , which is significantly higher than the specific (mass) heat capacity of other common metals, such as copper and iron.
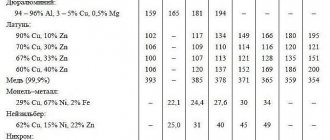
Below is a comparative table of the specific heat capacities of these metals. The heat capacity values in the table are in the temperature range from -223 to 927°C.
According to the table, it can be seen that the value of the specific heat capacity of aluminum is significantly higher than the value of this property for copper and iron , therefore this property of aluminum, such as the ability to accumulate heat well, is widely used in industry and heating engineering, making this metal irreplaceable.
Comparative table of heat capacity of aluminum, copper and iron Heat capacity of metal, J/(kg deg)
| t, °С | Aluminum Al | Copper Cu | Iron Fe |
| -173 | 483,6 | — | 216,1 |
| -73 | 800,2 | — | 385 |
| 27 | 903,7 | 385 | 450 |
| 127 | 951,3 | 397,7 | 491,1 |
| 227 | 991,8 | 408 | 530,7 |
| 327 | 1036,7 | 416,9 | 573,1 |
| 427 | 1090,2 | 425,1 | 619,9 |
| 527 | 1153,8 | 432,9 | 679,1 |
| 627 | 1228,2 | 441,7 | 772,8 |
| 727 | 1176,7 | 451,4 | 975,1 |
| 827 | 1176,7 | 464,3 | 794,1 |
| 927 | 1176,7 | 480,8 | 607,1 |
Sources:
from here
Thermophysical properties of aluminum alloys AMts, AMg, D16, AK, etc.
The table presents the composition and thermophysical properties of aluminum alloys for cold-worked, hardened and annealed states of the alloy:
- density of alloys, kg/m3;
- thermal conductivity coefficient, W/(m deg);
- coefficient of linear thermal expansion, 1/deg;
- specific electrical resistance, Ohm m.
Thermophysical properties are presented for the following aluminum alloys: A, AMts, AMg, Amg1, AMg5, AB, D18, D1, D16, AK8, AK4, 32S, V95. The properties of the alloys are given at room temperature, with the exception of the coefficient of thermal expansion (CTE), which is indicated for the temperature ranges of 20-100, 20-200 and 20-300°C.
Thermal conductivity of aluminum alloys
A summary table of the thermal conductivity of aluminum alloys is presented. It shows the thermal conductivity values of common aluminum alloys (aluminum alloys with silicon, copper, magnesium and zinc, casting alloys, duralumin) at various temperatures in the range from 4 to 700K.
According to the table, it can be seen that the thermal conductivity of aluminum alloys mainly increases with increasing temperature. An alloy such as AD1 has the greatest thermal conductivity at room temperature - its thermal conductivity at this temperature is 210 W/(m deg). Lower thermal conductivity is characteristic mainly of cast aluminum alloys, for example AK4, AL1, AL8 and others.
The temperature in the table is in degrees Kelvin!
Table of thermal conductivity of aluminum alloysAluminum alloyTemperature, KThermal conductivity of aluminum alloy, W/(m deg)
| AB | 298…373…473…573 | 176…180…184…189 |
| AD1 cold-hardened | 4…10…20…40…80…150…300 | 50…130…260…400…250…220…210 |
| AD31 hardened, aged | 4…10…20…40…80…200…300…600 | 35…87…170…270…230…200…190…190 |
| AD33 | 300…373…473…573 | 140…151…163…172 |
| AD35 | 298…373…473…573 | 170…174…178…182 |
| AK4 | 300…500…600…700 | 145…160…170…170 |
| AK6 hardened, aged | 20…77…223…293…373…473…573…673 | 35…90…192…176…180…184…184…189 |
| AK8 hardened, aged | 20…40…80…150…300…573…673 | 50…72…100…125…160…180…180 |
| AL1 | 300…400…600 | 130…140…150 |
| AL2 | 20…77…293 | 10…18…160 |
| AL4 | 300…473…673 | 150…160…155 |
| AL5 | 300…473…573 | 160…170…180 |
| AL8 | 300…473…673 | 92…100…110 |
| AMg1 | 298…373…473…573…673 | 184…188…192…188…188 |
| AMg2 | 4…10…20…40…80…150…300…373…473…573…673 | 4,6…12…25…49…77…100…155…159…163…164…167 |
| AMg3 | 20…77…90…203…293 | 41…86…89…123…132 |
| AMg5 annealed | 10…20…40…80…150…300…473…673 | 10…20…40…66…92…130…130…150 |
| AMg6 | 20…77…173…293 | 13…43…75…92 |
| AMts cold-worked | 4…10…20…40…80…150…300…473…573…673 | 11…28…58…110…140…150…180…180…184…188 |
| B93 | 300…473…673 | 160…170…160 |
| B95 | 300…473…673 | 155…160…160 |
| VAD1 | 20…80…300 | 30…61…160 |
| VAL1 | 300…473…673 | 130…150…160 |
| VAL5 | 300…573…673 | 150…160…160 |
| VD17 | 300…673 | 130…170 |
| D1 | 298…373…473…573…673 | 117…130…150…172…176 |
| D16 hardened, aged | 10…20…40…80…150…300…373…473…573 | 9…19…37…61…90…120…130…146…163 |
| D20 hardened, aged | 20…40…80…150…300…373…473…573…673 | 27…38…61…85…140…142…147…155…160 |
| D21 | 298…373…473…573 | 130…138…151…168 |
Properties of aluminum alloys with silicon, copper, magnesium and zinc
The table presents the composition and the following thermophysical properties of aluminum alloys:
- density of alloys, kg/m3;
- thermal conductivity coefficient, W/(m °C);
- coefficient of linear thermal expansion, 1/deg;
- corrosion resistance in water and air;
- temperature of strength change.
Main areas of use
In conclusion, we present the most common areas of use of the substance:
- Aviation kerosene. This is the name of motor fuel for gas turbine engines, which are equipped with various aircraft. These are kerosene fractions of direct distillation of oil. They are often hydrotreated and additives are added to improve performance properties. In Russia, five varieties of such fuel are produced for subsonic aviation - TS-1, T-1, T-1S, T-2 and RT, and for supersonic aviation - two (T-6 and T-8V).
- Rocket kerosene. Here this petroleum product acts as a hydrocarbon, environmentally friendly fuel and the working fluid of hydraulic machines. Its use in rocket engines was proposed back in 1914 by Tsiolkovsky. Paired with liquid oxygen, it is used in the lower stages of many launch vehicles.
- Technical kerosene. This is a raw material for the production of aromatic hydrocarbons, ethylene, propylene. In addition, it is the main fuel for firing porcelain and glass, and a solvent for washing parts and mechanisms.
- Lighting kerosene (KO-25, KO-30, KO-20, KO-22). It is used in lighting fixtures and is used as fuel for some kitchen stoves (kerosene stoves, kerosene stoves, kerosene gas). Another use is in heating. This is a solvent, a cleaning agent (widely used to remove residues of thermal pastes, various paints and varnishes), and a degreaser.
- Automotive kerosene. This application was characteristic of the dawn of the development of internal combustion engines. The petroleum product was widely used as fuel for carburetor and diesel internal combustion engines.
Among the non-trivial uses, the following can be distinguished: a folk remedy for getting rid of lice, treating head lice and diphtheria. In addition, kerosene helped get rid of bedbugs when wiping furniture with it.
As you have seen, kerosene immediately determines a complex of characteristics. And this seems natural given its multiple uses.
Application area
There are many industries where tungsten is used.
The main area of application is the production of alloys. This metal increases the hardness, strength, elasticity and improves the stretchability of various types of steel. It is usually prepared in two forms: Ferrotungsten is an alloy of iron and tungsten, it usually contains about 70-80% tungsten. Ferrotungsten is mixed with other metals and alloys (usually steel) to produce specialized compounds. And it is also produced in powder form. In the future, it is added to other metals in order to obtain new compounds with improved characteristics.
About 90% of all tungsten alloys are used in mining, construction, electrical and metalworking equipment. These alloys are used to make many things: heating elements in furnaces (due to their good thermal conductivity), parts for aircraft and spacecraft; equipment used in television, radar and radio engineering; high-strength drills; metal-cutting tools and similar equipment.
Small amounts of tungsten are used in incandescent lamps. The very thin wire that forms the filament in lamps is made from it. Electric current passes through this filament and heats it, which causes it to emit light. It does not melt due to the high melting point of tungsten.
It is also used in such devices and elements as:
- welding electrodes;
- counterweights;
- magnets;
- X-ray machines;
- windings and heating elements of electric stoves;
- cathodes of radio tubes and electronic devices (thoriated tungsten);
- magnetrons in microwave ovens;
- chemical catalysts.
It is also used in metalworking and mining, as well as in the production of pigments for paints.
Physical properties of kerosene:
| Parameter name: | Meaning: |
| Density of kerosene at 20 °C, g/cm3 (depends on the hydrocarbon composition, type and grade of kerosene)* | from 0.78 to 0.85 |
| Density of kerosene at 20 °C, kg/m3 (depending on the hydrocarbon composition, type and grade of kerosene)* | from 780 to 850 |
| Melting/freezing point (depending on the hydrocarbon composition and type of kerosene), °C | from -60 °C to -40 °C |
| Boiling point (depending on the hydrocarbon composition and type of kerosene), °C | from +150°C to +250°C |
| Kinematic viscosity at 20 °C (depending on the hydrocarbon composition, type and grade of kerosene), mm²/s | from 1.2 to 4.5% |
| Flash point** (depending on the hydrocarbon composition, type and grade of kerosene), °C | from +28 °С to +72 °С |
| Ignition temperature** (depends on the hydrocarbon composition, type and grade of kerosene), °C | from -10 °С to +105 °С |
| Self-ignition temperature (depending on the hydrocarbon composition, type and grade of kerosene), °C | 220 °C |
| Explosive concentrations of a mixture of kerosene with air (depending on the hydrocarbon composition, type and grade of kerosene), % by volume | from 0.6 to 8.0 |
| Specific heat of combustion of kerosene (depending on the hydrocarbon composition, type and grade of kerosene), mJ/kg | from 42.9 to 46.2 |
| Sulfur content (depending on the hydrocarbon composition, type and grade of kerosene), %% | no more than 1.0 |
Note:
* with increasing temperature, the density of kerosene decreases.
** ignition temperature is the temperature of a flammable substance at which it emits flammable vapors and gases at such a speed that, after ignition from the ignition source, stable combustion occurs;
** flash point - the temperature at which petroleum product vapors form a mixture with the surrounding air that flares up when a fire is brought to it.
Table of thermal conductivity of materials on Pli-
| Material | Density, kg/m3 | Thermal conductivity, W/(m deg) | Heat capacity, J/(kg deg) |
| Pressed paper plate | 600 | 0.07 | — |
| Cork plate | 80…500 | 0.043…0.055 | 1850 |
| Facing tiles, tiles | 2000 | 1.05 | — |
| Thermal insulation tile PMTB-2 | — | 0.04 | — |
| Alabaster slabs | — | 0.47 | 750 |
| Gypsum slabs GOST 6428 | 1000…1200 | 0.23…0.35 | 840 |
| Wood-fiber and particle boards (GOST 4598-74, GOST 10632-77) | 200…1000 | 0.06…0.15 | 2300 |
| Slabs made of expanded clay concrete | 400…600 | 0.23 | — |
| Polystyrene concrete slabs GOST R 51263-99 | 200…300 | 0.082 | — |
| Resol-formaldehyde foam boards (GOST 20916-75) | 40…100 | 0.038…0.047 | 1680 |
| Plates made of glass staple fiber with a synthetic binder (GOST 10499-78) | 50 | 0.056 | 840 |
| Slabs made of cellular concrete GOST 5742-76 | 350…400 | 0.093…0.104 | — |
| Reed slabs | 200…300 | 0.06…0.07 | 2300 |
| Silica slabs | 0.07 | — | |
| Flax insulating slabs | 250 | 0.054 | 2300 |
| Mineral wool slabs with bitumen binder grade 200 GOST 10140-80 | 150…200 | 0.058 | — |
| Mineral wool slabs with synthetic binder grade 200 GOST 9573-96 | 225 | 0.054 | — |
| Mineral wool slabs with synthetic bond (Finland) | 170…230 | 0.042…0.044 | — |
| Mineral wool slabs of increased rigidity GOST 22950-95 | 200 | 0.052 | 840 |
| Mineral wool slabs of increased rigidity with an organophosphate binder (TU 21-RSFSR-3-72-76) | 200 | 0.064 | 840 |
| Semi-rigid mineral wool slabs with starch binder | 125…200 | 0.056…0.07 | 840 |
| Mineral wool slabs with synthetic and bitumen binders | — | 0.048…0.091 | — |
| Soft, semi-rigid and hard mineral wool slabs with synthetic and bitumen binders (GOST 9573-82, GOST 10140-80, GOST 12394-66) | 50…350 | 0.048…0.091 | 840 |
| Foam plastic boards based on resol phenol-formaldehyde resins GOST 20916-87 | 80…100 | 0.045 | — |
| Expanded polystyrene boards GOST 15588-86 without pressing | 30…35 | 0.038 | — |
| Polystyrene foam plates (extrusion) TU 2244-001-47547616-00 | 32 | 0.029 | — |
| Perlite-bitumen slabs GOST 16136-80 | 300 | 0.087 | — |
| Perlite-fiber slabs | 150 | 0.05 | — |
| Perlite-phosphogel slabs GOST 21500-76 | 250 | 0.076 | — |
| Perlito-1 slabs Plastic concrete TU 480-1-145-74 | 150 | 0.044 | — |
| Perlite cement slabs | — | 0.08 | — |
| Construction slabs made of porous concrete | 500…800 | 0.22…0.29 | — |
| Thermobitumen thermal insulation slabs | 200…300 | 0.065…0.075 | — |
| Peat thermal insulation slabs (GOST 4861-74) | 200…300 | 0.052…0.064 | 2300 |
| Fiberboard slabs (GOST 8928-81) and wood concrete (GOST 19222-84) on Portland cement | 300…800 | 0.07…0.16 | 2300 |
Kerosene as fuel:
Kerosene (English kerosene from ancient Greek κηρός - “wax”) is a flammable mixture of liquid hydrocarbons (from C8 to C15) with a boiling point in the range of 150-250 °C, obtained by direct distillation or rectification of oil.
Externally, kerosene is a transparent, colorless (or slightly yellowish, or light brown), slightly oily liquid to the touch. Has a characteristic smell of petroleum products.
Kerosene is a flammable, flammable liquid. Refers to low-hazard substances and, in terms of the degree of impact on the human body, in accordance with GOST 12.1.007, belongs to the 4th hazard class. Combustible fuel.
Kerosene is lighter than water. Does not dissolve in water.
Kerosene forms explosive mixtures with air.
What is aviation fuel?
Fuel for use in the aviation industry is a flammable substance intended to be supplied mixed with air into the combustion chamber of an aircraft engine. The goal is to obtain thermal energy, which is released at the moment of oxidation of the mixture with oxygen, that is, combustion. The fuel poured into the coffered tanks of aircraft is divided into two types.
Aviation gasoline
This type of fuel is obtained using direct distillation, reforming or catalytic cracking. The main physical and chemical indicators of aviation gasoline are:
- resistance to detonation;
- chemical stability;
- factional composition.
Gasoline is characterized by high volatility and suitability for the formation of fuel-air mixtures necessary for current flight conditions.
This type of combustible mixture is used for combustion in piston internal combustion engines. Airplanes with such engines fly short distances on local airlines and are used for demonstration flights and air shows. The most popular brands in Russian small aviation were the brands of leaded gasoline for normal and lean mixtures, developed in the last quarter of the last century - B91/115 and B95/130. Today, the small aircraft fleet is fully fueled with regular AI-95 gasoline or imported AVGAS 100LL fuel.
Interesting: Why do children suck their thumb? Reasons, what to do, photos and videos
Aviation kerosene
Regular gasoline is not suitable for combustion in the combustion chamber of a turbojet aircraft engine. Piston engines use the effect of sudden ignition of the gasoline-air mixture to create a shock at the cylinder head. A completely different principle is used in jet engines
It is important here that the combustion is smooth. This is exactly what burning aviation kerosene provides
To fill the caissons of jet aircraft, fuel is used, which is obtained from the middle distillate kerosene fraction of oil with a boiling point of 150-280°C. 96-98% of the composition of aviation kerosene is naphthenic, paraffin and aromatic hydrocarbons. The rest of the composition comes from resins, nitrogenous and organometallic compounds.
Unit of measurement of specific heat capacity
Specific heat capacity is denoted by the letter $c$ .
The specific heat capacity of a substance is measured in $\frac{J}{kg \cdot \degree C}$ .
Let's consider this unit of measurement using graphite as an example. Its specific heat capacity is $750 \frac{J}{kg \cdot \degree C}$. What does this mean?
From this value we can say that:
- To heat a piece of graphite weighing $1 \space kg$ by $1 \degree C$ we need to expend an amount of heat equal to $750 \space J$
- When a piece of graphite weighing $1 \space kg$ is cooled by $1 \degree C$, an amount of heat will be released equal to $750 \space J$
- When the temperature of a piece of graphite weighing $1 \space kg$ changes by $1 \degree C$, it will either absorb or release an amount of heat equal to $750 \space J$