The chemical compound with the formula Al₄C₃ is called aluminum carbide. Its appearance is represented by a yellowish crystalloid substance. The compound is very resistant to environmental influences, the melting point of aluminum carbide is 1400 ° C, and the relative density is 2.36 g/cm³. The structural lattice of Al₄C₃ is complex; it contains carbon atoms. They appear there in the form of anions. Aluminum carbide, like other inorganic compounds, has many applications. This substance belongs to the first group, the difference of which is the unchanged valence, typical for the metal normally.
Aluminum carbide: obtaining the substance
The substance can be obtained by combining metal with carbon, which must be placed in an arc furnace. Calcium carbide also contains a small content of Al₄C₃. When producing electrolytes, the compound is a product of graphite corrosion. The reaction of aluminum oxide with carbon forms Al₄C₃. The next way to obtain this substance is to simultaneously calcinate coke and aluminum at a temperature of 1800 degrees. It is extracted by specially trained pyrotechnicians and chemists. You should not try to obtain aluminum carbide at home, or in laboratories not intended for this purpose.
Structure [edit]
Aluminum carbide has an unusual crystal structure, which consists of alternating layers of Al 2 C and Al 2 C 2. Each aluminum atom is coordinated to 4 carbon atoms, forming a tetrahedral arrangement. Carbon atoms exist in 2 different binding environments; one is a deformed octahedron of 6 Al atoms at a distance of 217 . The other is a distorted trigonal bipyramidal structure of 4 Al atoms at 190–194 pm and a fifth Al atom at 221 pm. [3] [4] Other carbides (IUPAC nomenclature: methides) also have complex structures.
Reactions[edit]
Aluminum carbide hydrolyzes to release methane. The reaction occurs at room temperature but accelerates rapidly when heated. [5]
Al 4 C 3 + 12 H 2 O → 4 Al (OH) 3 + 3 CH 4
Similar reactions occur with other proton reagents: [1]
Al 4 C 3 + 12 HCl → 4 AlCl 3 + 3 CH 4
Reactive hot isostatic pressing (tipping) at ≈40 MPa of appropriate mixtures of Ti, Al 4 C 3 graphite for 15 hours at 1300 °C produces predominantly single-phase samples of Ti 2 AlC 0.5 N 0.5, 30 hours at 1300 ° C. C produces predominantly single-phase Ti2AlC (titanium-aluminum carbide) samples. [6]
Physical and chemical properties
The main property of the substance is its ability to interact with water, oxygen and sodium hydroxide. In addition, aluminum carbide can melt, refract and dissolve. It has an enthalpy of formation ∆Н = -209 (S = 88.95), Gibbs energy = -196 t, molar heat capacity = 116.8. Refractive index of aluminum carbide = 2.7 for 20 degrees. Al₄C₃ can interact with many chemical elements, forming well-known compounds needed in industry. An example is one of the natural gases – methane. It can be obtained by mixing aluminum carbide with water. In this case, H2O acts as a solvent for the metal, as a result of which the main compound decomposes.
Aluminum. Chemistry of aluminum and its compounds
1. Position of aluminum in the periodic table of chemical elements 2. Electronic structure of aluminum 3. Physical properties 4. Occurrence in nature 5. Methods of preparation 6. Qualitative reactions 7. Chemical properties 7.1. Interaction with simple substances 7.1.1. Interaction with halogens 7.1.2. Interaction with sulfur 7.1.3. Interaction with phosphorus 7.1.4. Interaction with nitrogen 7.1.5. Interaction with carbon 7.1.6. Combustion 7.2. Interaction with complex substances 7.2.1. Interaction with water 7.2.2. Interaction with mineral acids 7.2.3. Interaction with sulfuric acid 7.2.4. Interaction with nitric acid 7.2.5. Interaction with alkalis 7.2.6. Interaction with oxidizing agents
Aluminum oxide 1. Methods of preparation 2. Chemical properties 2.1. Interaction with basic oxides 2.2. Interaction with bases 2.3. Interaction with water 2.4. Interaction with acid oxides 2.5. Interaction with acids 2.6. Interaction with reducing agents 2.7. Displacement of more volatile oxides from salts
Aluminum hydroxide 1. Methods of preparation 2. Chemical properties 2.1. Interaction with acids 2.2. Interaction with acid oxides 2.3. Interaction with alkalis 2.4. Heat decomposition
Aluminum salts
Binary aluminum compounds
Aluminum
Position in the periodic table of chemical elements
Aluminum is located in the main subgroup of group III (or in group 13 in the modern form of PSHE) and in the third period of the periodic table of chemical elements of D.I. Mendeleev.
Electronic structure of aluminum and properties
Electronic configuration of aluminum in the ground state :
+13Al 1s 2 2s22p63s23p1 1s 2s 2p 3s 3p
Electronic configuration of aluminum in excited state :
+13Al* 1s 2 2s22p63s13p2 1s 2s 2p 3s 3p
Aluminum exhibits paramagnetic properties. Aluminum in air quickly forms strong oxide films that protect the surface from further interaction, therefore it is resistant to corrosion .
Physical properties
Aluminum is a lightweight silver-white metal that can be easily formed, cast, and machined. Has high thermal and electrical conductivity.
Melting point 660°C, boiling point 1450°C, aluminum density 2.7 g/cm3.
Aluminum is one of the most valuable non-ferrous metals for recycling. Over the past years, the price of aluminum scrap at collection points has been steadily increasing. Follow the link to learn how to donate scrap aluminum.
Being in nature
Aluminum is the most abundant metal in nature, and the 3rd most abundant of all elements (after oxygen and silicon). The content in the earth's crust is about 8%.
In nature, aluminum occurs in the form of compounds:
Bauxite Al2O3 H2O
(with impurities of
SiO 2, Fe2O3, CaCO3)
- aluminum oxide hydrate.
Corundum Al2O3. Red corundum is called ruby, blue corundum is called sapphire.
Methods of obtaining
Aluminum
forms a strong chemical bond with oxygen.
Therefore, traditional methods for producing aluminum by reduction from oxide require large amounts of energy. For the industrial production of aluminum, the Hall-Heroux process is used. To lower the melting point, aluminum oxide is dissolved in molten cryolite (at a temperature of 960-970 ° C) Na3AlF6, and then subjected to electrolysis with carbon electrodes .
When dissolved in cryolite melt, aluminum oxide breaks down into ions: Al2O3 → Al3+ + AlO33-
Reduction of aluminum ions occurs at the cathode :
Cathode: Al3+ +3e → Al0
of aluminate ions occurs at the anode :
Anode: 4AlO33- – 12e → 2Al2O3 + 3O2
The overall equation for the electrolysis of molten aluminum oxide is:
2Al2O3 → 4Al + 3O2
Laboratory method
Aluminum production involves the reduction of aluminum from anhydrous aluminum chloride with potassium metal:
AlCl3 + 3K → Al + 3KCl
Qualitative reactions
A qualitative reaction to aluminum ions is the interaction of excess aluminum salts with alkalis . This produces a white amorphous precipitate of aluminum hydroxide .
For example , aluminum chloride reacts with sodium hydroxide :
AlCl3 + 3NaOH → Al(OH)3 + 3NaCl
With further addition of alkali, amphoteric aluminum hydroxide dissolves to form tetrahydroxoaluminate :
Al(OH)3 + NaOH = Na[Al(OH)4]
Please note that if we place an aluminum salt in an excess of alkali solution , then a white precipitate of aluminum hydroxide will not form, because in excess of alkali, aluminum compounds immediately transform into a complex :
AlCl3 + 4NaOH = Na[Al(OH)4] + 3NaCl
Aluminum salts can be detected using an aqueous ammonia solution. When soluble aluminum salts interact with an aqueous ammonia solution, a translucent gelatinous precipitate of aluminum hydroxide also forms.
AlCl3 + 3 NH3 H2O = Al(OH)3 ↓ + 3NH4Cl
Al3+ + 3 NH3 H2O = Al(OH)3 ↓ + 3NH4+
A video experience of the interaction of an aluminum chloride solution with an ammonia solution can be viewed here.
Chemical properties
1. Aluminum is a strong reducing agent . Therefore, it reacts with many nonmetals .
1.1. Aluminum reacts with halogens to form halides :
2Al + 3I2 → 2AlI3
1.2. Aluminum reacts with sulfur to form sulfides :
2Al + 3S → Al2S3
1.3. Aluminum reacts with phosphorus . In this case, binary compounds are formed - phosphides :
Al + P → AlP
1.4. Aluminum with nitrogen
reacts when heated to 1000°C to form
nitride :
2Al + N2 → 2AlN
1.5. Aluminum reacts with carbon to form aluminum carbide :
4Al + 3C → Al4C3
1.6. Aluminum reacts with oxygen to form an oxide :
4Al + 3O2 → 2Al2O3
A video experience of the interaction of aluminum with atmospheric oxygen (combustion of aluminum in air) can be viewed here.
2. Aluminum interacts with complex substances:
2.1. Does aluminum with water ? You can easily find the answer to this question if you delve a little into your memory. Surely at least once in your life you have come across aluminum pans or aluminum cutlery. This is the question I liked to ask students during exams. What is most surprising is that I received different answers - for some, aluminum did react with water. And very, very many people gave up after the question: “Maybe aluminum reacts with water when heated?” When heated, aluminum reacted with water in half of the respondents))
However, it is easy to understand that aluminum still does not interact with water under normal conditions (and even when heated) . And we have already mentioned why: due to the formation of an oxide film . But if aluminum is cleaned from the oxide film (for example, amalgamated ), then it will interact with water very actively with the formation of aluminum hydroxide and hydrogen :
2Al0 + 6H2+O → 2Al+3(OH)3 + 3H20
Aluminum amalgam can be obtained by keeping pieces of aluminum in a solution of mercury (II) chloride:
3HgCl2 + 2Al → 2AlCl3 + 3Hg
A video experience of the interaction of aluminum amalgam with water can be viewed here.
2.2. Aluminum reacts with mineral acids (hydrochloric, phosphoric and dilute sulfuric acid). This produces salt and hydrogen.
For example , aluminum reacts violently with hydrochloric acid :
2Al + 6HCl = 2AlCl3 + 3H2↑
2.3. Under normal conditions, aluminum does not react with concentrated sulfuric acid due to passivation - the formation of a dense oxide film. When heated, the reaction occurs and sulfur (IV) oxide , aluminum sulfate and water :
2Al + 6H2SO4(conc.) → Al2(SO4)3 + 3SO2 + 6H2O
2.4. Aluminum does not react with concentrated nitric acid also due to passivation.
with dilute nitric acid to form molecular nitrogen :
10Al + 36HNO3 (dil) → 3N2 + 10Al(NO3)3 + 18H2O
When aluminum in powder form reacts with very dilute nitric acid, ammonium nitrate can form :
8Al + 30HNO3(highly diluted) → 8Al(NO3)3 + 3NH4NO3 + 9H2O
2.5. Aluminum is an amphoteric metal, so it reacts with alkalis . When aluminum reacts with alkali solution tetrahydroxoaluminate and hydrogen :
2Al + 2NaOH + 6H2O → 2Na[Al(OH)4] + 3H2 ↑
A video of the interaction of aluminum with alkali and water can be viewed here.
Aluminum reacts with alkali to form aluminate and hydrogen :
2Al + 6NaOH → 2Na3AlO3 + 3H2 ↑
The same reaction can be written in another form (in the Unified State Examination I recommend writing the reaction in this form):
2Al + 6NaOH → 2NaAlO2 + 3H2↑ + 2Na2O
2.6. Aluminum reduces less active metals from oxides . The process of reducing metals from oxides is called aluminothermy .
For example , aluminum is replacing copper
from
copper(II) oxide. The reaction is very exothermic:
2Al + 3CuO → 3Cu + Al2O3
Another example : aluminum reduces iron from iron scale , iron (II, III) oxide :
8Al + 3Fe3O4 → 4Al2O3 + 9Fe
The reducing properties of aluminum also appear when it interacts with strong oxidizing agents: sodium peroxide , nitrates and nitrites in an alkaline environment, permanganates , chromium (VI) compounds :
2Al + 3Na2O2 → 2NaAlO2 + 2Na2O
8Al + 3KNO3 + 5KOH + 18H2O → 8K[Al(OH)4] + 3NH3
10Al + 6KMnO4 + 24H2SO4 → 5Al2(SO4)3 + 6MnSO4 + 3K2SO4 + 24H2O
2Al + NaNO2 + NaOH + 5H2O → 2Na[Al(OH)4] + NH3
Al + 3KMnO4 + 4KOH → 3K2MnO4 + K[Al(OH)4]
4Al + K2Cr2O7 → 2Cr + 2KAlO2 + Al2O3
Aluminium oxide
Methods of obtaining
Aluminum oxide can be obtained by various methods:
1. Combustion of aluminum in air:
4Al + 3O2 → 2Al2O3
2. Decomposition of aluminum hydroxide when heated:
2Al(OH)3 → Al2O3 + 3H2O
3. Aluminum oxide can be obtained by decomposition of aluminum nitrate :
4Al(NO3)3 → 2Al2O3 + 12NO2 + 3O2
Chemical properties
Aluminum oxide is a typical amphoteric oxide . Interacts with acidic and basic oxides, acids, alkalis.
1. When aluminum oxide interacts with basic oxides, aluminate salts are formed .
For example , aluminum oxide reacts with sodium oxide :
Na2O + Al2O3 → 2NaAlO2
2. Aluminum oxide reacts with soluble bases (alkalis). In this case, salts are formed in the melt - aluminates, and in the solution - complex salts . In this case, aluminum oxide exhibits acidic properties .
For example , aluminum oxide reacts with sodium hydroxide
in the melt with the formation of
sodium aluminate and water :
2NaOH + Al2O3 → 2NaAlO2 + H2O
Aluminum oxide dissolves in excess alkali to form tetrahydroxyaluminate :
Al2O3 + 2NaOH + 3H2O → 2Na[Al(OH)4]
3. Aluminum oxide does not react with water.
4. Aluminum oxide interacts with acidic oxides (strong acids). In this case, aluminum salts In this case, aluminum oxide exhibits basic properties .
For example , aluminum oxide reacts with sulfur(VI) oxide to form aluminum sulfate:
Al2O3 + 3SO3 → Al2(SO4)3
5. Aluminum oxide reacts with soluble acids to form medium and acid salts .
For example , aluminum oxide reacts with sulfuric acid :
Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
6. Aluminum oxide exhibits weak oxidizing properties .
For example , aluminum oxide reacts with calcium hydride to produce aluminum , hydrogen and calcium oxide :
Al2O3 + 3CaH2 → 3CaO + 2Al + 3H2
Electric current reduces aluminum from oxide (aluminum production):
2Al2O3 → 4Al + 3O2
7. Aluminum oxide - solid, non-volatile. Consequently, it displaces more volatile oxides (usually carbon dioxide) from the salts during fusion.
For example , from sodium carbonate :
Al2O3 + Na2CO3 → 2NaAlO2 + CO2
Aluminum hydroxide
Methods of obtaining
1. Aluminum hydroxide can be obtained by the action of an ammonia on aluminum salts .
For example , aluminum chloride reacts with an aqueous solution of ammonia to form aluminum hydroxide and ammonium chloride :
AlCl3 + 3NH3 + 3H2O = Al(OH)3 + 3NH4Cl
2. By passing carbon dioxide , sulfur dioxide or hydrogen sulfide through a solution of sodium tetrahydroxyaluminate:
Na[Al(OH)4] + CO2 = Al(OH)3 + NaHCO3
To understand how this reaction proceeds, you can use a simple technique: mentally break down the complex substance Na[Al(OH)4] into its component parts: NaOH and Al(OH)3. Next, we determine how carbon dioxide reacts with each of these substances and record the products of their interaction. Because Al(OH)3 does not react with CO2, then we write Al(OH)3 on the right without change.
3. Aluminum hydroxide can be obtained by the action of a lack of alkali on an excess of aluminum salt .
For example , aluminum chloride reacts with a lack of potassium hydroxide to form aluminum hydroxide and potassium chloride :
AlCl3 + 3KOH(insufficient) = Al(OH)3↓+ 3KCl
4. Also, aluminum hydroxide is formed by the interaction of soluble aluminum salts with soluble carbonates, sulfites and sulfides . Aluminum sulfides, carbonates and sulfites are irreversibly hydrolyzed in aqueous solution.
For example: aluminum bromide reacts with sodium carbonate . In this case, a precipitate of aluminum hydroxide precipitates, carbon dioxide is released and sodium bromide is formed:
2AlBr3 + 3Na2CO3 + 3H2O = 2Al(OH)3↓ + 3CO2↑ + 6NaBr
Aluminum chloride reacts with sodium sulfide to form aluminum hydroxide, hydrogen sulfide and sodium chloride:
2AlCl3 + 3Na2S + 6H2O = 2Al(OH)3 + 3H2S↑ + 6NaCl
Chemical properties
1. Aluminum hydroxide reacts with soluble acids . In this case medium or acidic salts , depending on the ratio of reagents and the type of salt.
For example , aluminum hydroxide reacts with nitric acid to form aluminum nitrate
:
Al(OH)3 + 3HNO3 → Al(NO3)3 + 3H2O
Al(OH)3 + 3HCl → AlCl3 + 3H2O
2Al(OH)3 + 3H2SO4 → Al2(SO4)3 + 6H2O
Al(OH)3 + 3HBr → AlBr3 + 3H2O
2. Aluminum hydroxide reacts with acidic oxides of strong acids .
For example , aluminum hydroxide reacts with sulfur(VI) oxide to form aluminum sulfate
:
2Al(OH)3 + 3SO3 → Al2(SO4)3 + 3H2O
3. Aluminum hydroxide reacts with soluble bases (alkalis). In this case, salts are formed in the melt - aluminates, and in the solution - complex salts . In this case, aluminum hydroxide exhibits acidic properties .
For example , aluminum hydroxide reacts with potassium hydroxide
in the melt with the formation of
potassium aluminate and water :
KOH + Al(OH)3 → KAlO2 + 2H2O
Aluminum hydroxide dissolves in excess alkali to form tetrahydroxyaluminate :
Al(OH)3 + KOH → K[Al(OH)4]
4. Aluminum hydroxide decomposes when heated:
2Al(OH)3 → Al2O3 + 3H2O
A video experience of the interaction of aluminum hydroxide with hydrochloric acid and alkalis (amphoteric properties of aluminum hydroxide) can be viewed here.
Aluminum salts
Aluminum nitrate and sulfate
Aluminum nitrate, when heated, decomposes into aluminum oxide , nitrogen (IV) oxide and oxygen :
4Al(NO3)3 → 2Al2O3 + 12NO2 + 3O2
Aluminum sulfate, when heated strongly, decomposes in a similar way - into aluminum oxide , sulfur dioxide and oxygen :
2Al2(SO4)3 → 2Al2O3 + 6SO2 + 3O2
Complex aluminum salts
To describe the properties of complex aluminum salts - hydroxyaluminates , it is convenient to use the following technique: mentally break tetrahydroxoaluminate into two separate molecules - aluminum hydroxide and alkali metal hydroxide.
For example , we break sodium tetrahydroxyaluminate into aluminum hydroxide and sodium hydroxide:
Na[Al(OH)4] into NaOH and Al(OH)3
The properties of the entire complex can be determined as the properties of these individual compounds.
Thus, aluminum hydroxo complexes react with acidic oxides .
For example , the hydroxo complex is destroyed by excess carbon dioxide . In this case, NaOH reacts with CO2 to form an acid salt (with an excess of CO2), and amphoteric aluminum hydroxide does not react with carbon dioxide, therefore, it simply precipitates:
Na[Al(OH)4] + CO2 → Al(OH)3↓ + NaHCO3
Similarly, potassium tetrahydroxyaluminate reacts with carbon dioxide:
K[Al(OH)4] + CO2 → Al(OH)3 + KHCO3
By the same principle, tetrahydroxoaluminates react with sulfur dioxide SO2:
Na[Al(OH)4] + SO2 → Al(OH)3↓ + NaHSO3
K[Al(OH)4] + SO2 → Al(OH)3 + KHSO3
But under the influence of an excess of strong acid, a precipitate does not form, because amphoteric aluminum hydroxide reacts with strong acids.
For example , with hydrochloric acid :
Na[Al(OH)4] + 4HCl(excess) → NaCl + AlCl3 + 4H2O
True, under the influence of a small amount ( lack ) of a strong acid , a precipitate will still form; there will not be enough acid to dissolve aluminum hydroxide:
Na[Al(OH)4] + НCl(deficiency) → Al(OH)3↓ + NaCl + H2O
Similarly, with a lack of nitric acid, aluminum hydroxide precipitates:
Na[Al(OH)4] + HNO3(deficiency) → Al(OH)3↓ + NaNO3 + H2O
The complex is destroyed when interacting with chlorine water (aqueous solution of chlorine) Cl2:
2Na[Al(OH)4] + Cl2 → 2Al(OH)3↓ + NaCl + NaClO + H2O
In this case, chlorine disproportionates .
The complex can also react with excess aluminum chloride . In this case, a precipitate of aluminum hydroxide precipitates:
AlCl3 + 3Na[Al(OH)4] → 4Al(OH)3↓ + 3NaCl
If you evaporate water from a solution of a complex salt and heat the resulting substance, you will be left with the usual aluminate salt:
Na[Al(OH)4] → NaAlO2 + 2H2O↑
K[Al(OH)4] → KAlO2 + 2H2O
Hydrolysis of aluminum salts
Soluble aluminum salts and strong acids hydrolyze at the cation . Hydrolysis proceeds stepwise and reversibly , i.e. a little:
Stage I: Al3+ + H2O = AlOH2+ + H+
Stage II: AlOH2+ + H2O = Al(OH)2+ + H+
Stage III: Al(OH)2+ + H2O = Al(OH)3 + H+
However, aluminum carbonates and their acid salts are hydrolyzed irreversibly , completely , i.e. do not exist in an aqueous solution, but are decomposed by water :
Al2(SO4)3 + 6NaHSO3 → 2Al(OH)3 + 6SO2 + 3Na2SO4
2AlBr3 + 3Na2CO3 + 3H2O → 2Al(OH)3↓ + CO2↑ + 6NaBr
2Al(NO3)3 + 3Na2CO3 + 3H2O → 2Al(OH)3↓ + 6NaNO3 + 3CO2↑
2AlCl3 + 3Na2CO3 + 3H2O → 2Al(OH)3↓ + 6NaCl + 3CO2↑
Al2(SO4)3 + 3K2CO3 + 3H2O → 2Al(OH)3↓ + 3CO2↑ + 3K2SO4
2AlCl3 + 3Na2S + 6H2O → 2Al(OH)3 + 3H2S↑ + 6NaCl
You can read more about hydrolysis in the corresponding article.
Aluminates
Salts in which aluminum is an acidic residue (aluminates) are formed from aluminum oxide when fused with alkalis and basic oxides:
Al2O3 + Na2O → 2NaAlO2
To understand the properties of aluminates, it is also very convenient to break them down into two separate substances.
For example, we mentally divide sodium aluminate into two substances: aluminum oxide and sodium oxide.
NaAlO2 into Na2O and Al2O3
Then it will become obvious to us that aluminates react with acids to form aluminum salts :
KAlO2 + 4HCl → KCl + AlCl3 + 2H2O
NaAlO2 + 4HCl → AlCl3 + NaCl + 2H2O
NaAlO2 + 4HNO3 → Al(NO3)3 + NaNO3 + 2H2O
2NaAlO2 + 4H2SO4 → Al2(SO4)3 + Na2SO4 + 4H2O
Under the influence of excess water, aluminates transform into complex salts:
KAlO2 + H2O = K[Al(OH)4]
NaAlO2 + 2H2O = Na[Al(OH)4]
Binary compounds
Aluminum sulfide under the action of nitric acid is oxidized to sulfate:
Al2 S3 + 8HNO3 → Al2(SO4)3 + 8NO2 + 4H2O
or to sulfuric acid (under the influence of hot concentrated acid ):
Al2 S3 + 30HNO3(conc.hor.) → 2Al(NO3)3 + 24NO2 + 3H2SO4 + 12H2O
Aluminum sulfide decomposes with water :
Al2S3 + 6H2O → 2Al(OH)3↓ + 3H2S↑
Aluminum carbide also decomposes with water when heated into aluminum hydroxide and methane :
Al4C3 + 12H2O → 4Al(OH)3 + 3CH4
Aluminum nitride decomposes under the action of mineral acids into aluminum and ammonium salts:
AlN + 4HCl → AlCl3 + NH4Cl
Also, aluminum nitride decomposes under the influence of water :
AlN + 3H2O → Al(OH)3↓ + NH3
Applications of aluminum carbide
Al₄C₃ is used in various types of industries. When aluminum carbide is produced using graphite particles, a very strong material is formed. Tools containing this compound have the same hardness as topaz. Typically, aluminum carbide is added to cutting objects used on machine tools, as medical equipment, etc. In addition, this compound is a necessary material in pyrotechnics. It has been used in this area for a long time, and so far no replacement has been found for it. Here aluminum carbide is necessary to produce sparks. Its presence in pyrotechnic works depends on what and in what quantity gunpowder is used. Another area of application of the substance is the chemical industry, where the compound is necessary for the formation of various products, in particular organic gases.
Examples of problem solving
| Exercise | The molar mass of the nitrogen-hydrogen compound is 32 g/mol. Determine the molecular formula of a substance whose mass fraction of nitrogen is 85.7%. |
| Solution | The mass fraction of element X in a molecule of the composition NX is calculated using the following formula: |
Let's calculate the mass fraction of hydrogen in the compound:
ω(H) = 100% - ω(N) = 100% - 85.7% = 14.3%.
Let us denote the number of moles of elements included in the compound as “x” (nitrogen), “y” (hydrogen). Then, the molar ratio will look like this (the values of relative atomic masses taken from D.I. Mendeleev’s Periodic Table are rounded to whole numbers):
This means that the simplest formula for combining nitrogen with hydrogen will be NH2 and a molar mass of 16 g/mol [M(NH2) = Ar(N) + 2×Ar(H) = 14+ 2×1 = 14 + 2 = 16 g/mol ].
To find the true formula of an organic compound, we find the ratio of the resulting molar masses:
This means that the indices of nitrogen and hydrogen atoms should be 2 times higher, i.e. the formula of the substance will be N2H4. This is hydrazine.
| Exercise | Establish the mass formula of a substance containing 26.5% potassium, 35.4% chromium and 38.1% oxygen. |
| Solution | The mass fraction of element X in a molecule of the composition NX is calculated using the following formula: |
Let us denote the number of moles of elements included in the compound as “x” (potassium), “y” (chromium) and “z” (oxygen). Then, the molar ratio will look like this (the values of relative atomic masses taken from D.I. Mendeleev’s Periodic Table are rounded to whole numbers):
x:y:z = ω(K)/Ar(K) : ω(Cr)/Ar(Cr) : ω(O)/Ar(O);
This means that the formula for the compound of potassium, chromium and oxygen will be KCrO4. This is potassium chromate.
Source
What is methane?
This compound belongs to the organic group; its structure is a simple hydrocarbon and has the formula CH4. Methane reacts little with water and is odorless and colorless. The gas belongs to the group of alkanes and is quite resistant to various chemical reactions. It is believed that methane is not dangerous to human health, however, some scientists claim the opposite. People who have constant contact with the gas experience changes in the central nervous system. Methane is explosive, so in industrial production it is necessary to carefully monitor its concentration in the air. Due to the fact that the gas is odorless, it is quite difficult to notice a leak. For this reason, enterprises install special sensors that record its level.
Physical properties
When choosing almost any material, you should pay most attention to physical properties. For the one under consideration they are as follows:
- The compound has a crystalline structure.
- The melting point is 2300 °C. It is worth considering that such a figure is characteristic only of the pure composition. The addition of various impurities to the composition causes the melting point to drop significantly.
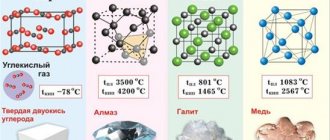
It is worth considering that calcium carbide in most cases is in a solid state. In addition, the color can vary from gray to brown. The physical properties of calcium carbide determine its widespread use in a wide variety of industries.
Methods for producing methane
Due to the fact that gas is a natural compound, it is not always produced in the laboratory.
Methane is produced under anaerobic conditions; this occurs as a result of fermentation processes in swamps, animal intestines, and overly wet soil. According to scientists, one of Saturn's moons contains liquid mixtures containing this gas on its surface. Methane is also one of the constituent parts of the atmospheres of large planets. The highest methane content is observed in natural, mine and swamp gases. In industrial conditions, gas is produced by hydrogenation and coking of coal. Methane production is also carried out in laboratories. One way to make it is by heating acetic acid and sodium hydroxide (or lime). The latter substance is also combined with acetate as a result of melting, which also results in the formation of methane. Both methods do not require the presence of water. The third method of producing gas is hydrolysis, which aluminum carbide undergoes. In this case, methane is formed faster. The hydrolysis method is also less expensive since it does not require exposure to high temperatures.
CARBIDES, occupational hazards
CARBIDES, occupational hazards
,—carbon compounds with metals and certain non-metals that can have an adverse effect on the human body. Natural crystals are found in deep rocks of the earth's crust, sometimes in meteorites. Get K. Ch. arr. heating metal and carbon powders (coal, soot) in an inert or reducing gas environment. C. are used in nuclear engineering, astronautics, electronics, high-temperature technology, mechanical engineering, metallurgy, as well as for the production of acetylene (see Acetylene) and cyanamide (see Defoliants).
Based on the nature of their interaction with water, they are divided into degradable and nondegradable. The alkali and alkaline earth metals (calcium, strontium, barium), as well as magnesium, beryllium, aluminum, lanthanides, and actinides, are classified as decomposed by water. The covalent compounds of boron and silicon, as well as the compounds of d-transition metals of groups IV–VIII of D. I. Mendeleev’s periodic table, are classified as non-water-degradable.
In carbon production, dust may be released from the starting components, the finished product, and gases (carbon monoxide). There is intense heat generation.
Dust from water-decomposed calcium (especially CaC2) causes irritation of the mucous membrane of the respiratory tract, especially the nasal cavity, as well as the mucous membranes of the eye. Additional toxic effects are caused by inhalation of acetylene. When it comes into contact with skin, especially wet skin, K. dust causes irritation, ulceration and an inflammatory reaction. When receiving and using potassium calcium, measures are taken to prevent the entry of dust, acetylene and other gases into the air. It is necessary to protect the skin and mucous membranes when working with water-degradable compounds.
In the production of products from carbon, powder or plasma metallurgy technology is used. Products made from carbon are processed using abrasive, ultrasonic, or electric spark methods. During crushing of mixtures, weighing of charge components, sifting, loading and unloading, packaging, and processing, a disintegration aerosol is formed. When servicing electric arc furnaces, low-frequency noise is recorded (see Noise). When casting molten carbon, hydrocarbon vapors and carbon monoxide may be released.
Applications of aluminum carbide in industrial chemistry
In addition to hydrolyzing the compound, it can also be used as a reagent. This use of aluminum carbide is necessary for determining the content of certain substances. In particular, this way you can detect a valuable gas - tritium, which is present in water. In addition, a combination salt can be obtained from aluminum carbide. It is obtained, like methane, by combining a substance with water. This salt is called sodium tetrahydroxoaluminate. It is necessary for coloring fabrics. The production and use of carbide are very important for various industries, so the costs of its production are quite high. Funds for the purchase of the substance are allocated by the state.
Chemical properties of aluminum carbide. Chemical reactions of aluminum carbide:
The chemical properties of aluminum carbide are similar to those of other metal carbides. Therefore, it is characterized by the following chemical reactions:
1. reaction of aluminum carbide and hydrogen:
The reaction produces aluminum and methane.
2. reaction of aluminum carbide and oxygen:
As a result of the reaction, aluminum oxide and carbon monoxide (IV) are formed.
3. reaction of aluminum carbide and chlorine:
The reaction produces aluminum chloride and carbon (IV) chloride (carbon tetrachloride).
4. reaction of aluminum carbide, sodium hydroxide and water:
As a result of the reaction, sodium tetrahydroxoaluminate and methane are formed.
5. reaction of aluminum carbide and nitric acid:
The reaction produces aluminum nitrate and methane.
Similar reactions of aluminum carbide occur with other acids.
6. decomposition reaction of aluminum carbide (reaction of aluminum carbide and water):
As a result of the decomposition reaction of aluminum carbide (the reaction of aluminum carbide and water), aluminum hydroxide and methane are formed. This reaction is a laboratory method for producing methane.
7. thermal decomposition reaction of aluminum carbide:
Al4C3 → 4Al + 3C (t > 2200 o C).
The thermal decomposition reaction of aluminum carbide produces aluminum and carbon.
What is carbide?
Homemade bombs. This is what comes to mind first when we hear the word carbide . And no, the production of these dangerous toys was not carried out by defense industry enterprises, but, as a rule, by boys, about ten years old.
Twenty years ago it was a favorite pastime among teenagers. Now everyone sits at their tablets, but back then the world was ruled by the inquisitive mind of a child who strove to try everything in practice.
In order to feel like Rimbaud, you needed to get one miracle stone. Children most often found them at construction sites. And then everything was simple: a plastic vessel, a stone, water, a tightly screwed cap. All this was zealously shaken, and at best, thrown away. And in the worst case, the “shell” exploded right in the hands, then injuries could not be avoided.
Read also: Aluminum is an alloy or metal
There were also safer ways to use the find, for example, simply throwing it into a puddle, then you could observe something similar to the effect of modern bath bombs. So what kind of popular “toy” is this? Most of us believed that nature produced carbide as we know it. But actually it is not. And today you will see this.
So, this substance is always very hard, plus, in order to melt it, you need to make a remarkable effort. They look like dark, light, greenish stones, or powder, it all depends on the composition. Its shelf life is short, usually six months. It will not be possible to place containers in a general warehouse; such potentially dangerous substances must have their own compartment.
As you already know, carbide constantly strives to explode. Moreover, some connections do not even need special conditions. It is enough just to pour the powder from container to container, and it can suddenly explode.
Being in nature
Natural aluminum consists almost entirely of a single stable isotope, 27Al, with traces of 26Al, a radioactive isotope with a half-life of 720 thousand years, formed in the atmosphere when bombarded by argon
cosmic ray protons.
In terms of prevalence in nature, it ranks 1st among metals and 3rd among elements, second only to oxygen and silicon. The percentage of aluminum content in the earth's crust, according to various researchers, ranges from 7.45 to 8.14% of the mass of the earth's crust.
In nature, aluminum is found only in compounds (minerals). Some of them:
- Bauxite - Al2O3 • H2O (with admixtures of SiO2, Fe2O3, CaCO3)
- Nephelines - KNa3[AlSiO4]4
- Alunites - KAl(SO4)2 • 2Al(OH)3
- Alumina (mixtures of kaolins with sand SiO2, limestone CaCO3, magnesite MgCO3)
- Corundum - Al2O3
- Feldspar (orthoclase) – K2O×Al2O3×6SiO2
- Kaolinite – Al2O3×2SiO2 × 2H2O
- Alunite - (Na,K)2SO4×Al2(SO4)3×4Al(OH)3
- Beryl - 3BeO • Al2O3 • 6SiO2
Natural waters contain aluminum in the form of low-toxic chemical compounds, for example, aluminum fluoride. The type of cation or anion depends, first of all, on the acidity of the aqueous medium. Aluminum concentrations in surface water bodies in Russia range from 0.001 to 10 mg/l.
Transportation and storage
Calcium carbide powder decomposes almost instantly when exposed to moisture. This produces acetylene, which in high concentrations is flammable and explosive. That is why it is necessary to pay quite a lot of attention to the storage of calcium carbide, for which cans and special drums are often used. Other storage features include the following:
- The released acetylene is lighter than air, so it accumulates at the top. It is worth considering that it has narcotic effects and can spontaneously ignite.
- When producing large volumes of a substance, special attention is paid to safety precautions. Special packaging is used for packaging.
- To open the package, use tools that do not cause sparks.
- If the substance gets on the skin or mucous membrane, it must be removed immediately. In this case, the affected surface is treated with a special cream or other protective and healing substance.
- According to established rules, transportation can only be carried out using a covered vehicle. However, delivery by air is prohibited.
Transport container
Regulations also prohibit storing calcium carbide with other chemicals and heat sources. This is because the resulting gases can react chemically with other chemicals and ignite.