Fluxes for aluminum
Soldering aluminum is a rather complex technological process. In addition to the oxide film on the surface of aluminum, the process is complicated by the need to expose it to higher temperatures than when soldering copper. However, aluminum can be soldered because special fluxes have been developed for this purpose.
The main task of flux for low-temperature soldering of aluminum is to dissolve the oxide film, which interferes with the normal spreading of solder and joining of parts. For soldering aluminum, only active fluxes containing acid are used. Rosin and other similar inactive fluxes are absolutely not suitable for these purposes.
Why is aluminum difficult to solder?
Many people have tried to solder aluminum at home and understand well: the solder does not want to stick to the surface of the parts. This occurs due to the formation of a stable oxide film on the metal, which has low adhesion to the solder material. Methods for soldering aluminum at home come down to fighting the protective film.
In mineralogy, aluminum oxide is called corundum. It consists of transparent crystals, which are gemstones. Corundum has different colors depending on the impurities: chromium gives a reddish tint, sapphire gives a bluish tint. The oxide film is highly durable and cannot be soldered. It must be removed from the surface and then begin to solder the parts.
Soldering aluminum wires
During electrical installation, the metal cores of the cables are connected by soldering. This guarantees reliable contact at the commutation point. However, while it is easy to apply solder to copper wire, coating aluminum conductor is a little more difficult. In addition, it is impossible to directly connect two metals - they form a galvanic couple. When an electric current passes through the twist, it will heat up.
For your information!
When cleaning the core, it is important not to damage the base metal. Notched aluminum can break easily.
The difficulty of soldering lies in the fact that there is an oxide film on the surface of the metal that is difficult to remove. It is formed instantly when aluminum comes into contact with oxygen in the air. This is due to the high chemical activity of the metal. Without the protective film, it would completely react with oxygen. To ensure that the solder evenly covers the wire and holds tightly to it, its surface is cleaned by etching with fluxes or without access to air.
How to remove oxide film
The film is removed from the metal surface in several ways, the most effective being chemical and mechanical. Both methods require an airless environment in which there is no oxygen to work.
The chemical method is based on the deposition of zinc or copper on the surface of the workpiece by electrolysis. Copper sulfate in the form of a concentrated solution is applied to the area prepared for soldering. The negative terminal of a battery or other power source is attached to a clean area of metal. One end of the copper wire is connected to the positive terminal, the other is dipped into a solution on the surface of the aluminum. As a result of electrolysis, copper or zinc is deposited in a thin layer on the aluminum and adheres tightly to it. Now you can solder aluminum with tin.
An oil film is used to remove the oxide. For this method, it is better to use synthetic or transformer oil with a low water content. Other types of oils need to be kept at a temperature of +150...+200°C, the water will evaporate. At higher temperatures, the contents will begin to splatter. Dehydrated oil is applied to the surface of the aluminum part. Use sandpaper to rub the aluminum under the applied layer to remove the oxide.
The emery cloth is replaced with a scalpel, a serrated soldering iron tip, or iron filings obtained from a nail rubbed with a file. The shavings are poured onto the oil and the soldering iron tip is rubbed over the surface, removing the oxide layer. It is advisable to heat a massive part with a hot air stream. The solder is dipped into the oil droplet with a soldering iron and rubbed over the soldering area. To improve the soldering process, rosin or other flux is added.
Surface preparation
Before you begin tinning, you must complete the following steps:
- degrease the surface using acetone, gasoline or any other solvent;
- remove the oxide film from the place where soldering will be performed. For cleaning, use sandpaper, an abrasive wheel or a brush with steel wire bristles. As an alternative, etching can be used, but this procedure is not so common due to its specificity.
It should be taken into account that it will not be possible to completely remove the oxide film, since a new formation will immediately appear in the cleaned area. Therefore, stripping is carried out not with the goal of completely removing the film, but to reduce its thickness in order to simplify the task of the flux.
Heating the soldering area
To solder small parts, you can use a soldering iron with a power of at least 100W. Massive items will require a more powerful heating tool.
Soldering iron 300 Watt
The best option for heating is to use a gas burner or blowtorch.
Simple gas burner
When using a burner as a heating tool, the following nuances should be taken into account:
- Do not overheat the base metal, as it may melt. Therefore, it is necessary to regularly monitor the temperature during the process. This can be done by touching the solder to the heated element. Melting the solder will let you know that the required temperature has been reached;
- Oxygen should not be used to enrich the gas mixture, since it promotes strong oxidation of the metal surface.
Fluxes for aluminum soldering
Fluxes are highly active, so after soldering they need to be washed off with a solution of water and alkali. Baking soda works well as an alkali. After the alkali, the joint is washed with clean water. Respiratory organs should be protected from flux vapors entering them. They can irritate mucous membranes and enter the blood. The most common of them need to be considered each separately.
Rosin
Rosin is the most popular of all fluxes. It is used in joining various metals. It works on aluminum only in the absence of air, so it is rarely used. More time is spent when working with rosin, but less efficiency. This flux is not for professionals; it can perform soldering, but the quality of the connection is not strong.
Powder flux
Aluminum is soldered with a gas torch using powder fluxes. It is not recommended to add oxygen to the flame; it reduces the efficiency of the flux. The most common fluxes:
- F-34A;
- borax;
- acetylsalicylic acid;
- solder fat.
F-34A is an active flux containing 50% potassium chloride, 32% lithium chloride, 10% sodium fluoride and 8% zinc chloride. The composition is used with solders containing chemical additives. It is hygroscopic and dissolves in water.
Borax is a powder that melts at 700°C, is soluble in water, and can be washed off with an aqueous solution of citric acid. It is characterized by low cost.
Acetylsalicylic acid is found in the form of aspirin tablets. When heated by a soldering iron, vapors that are harmful to human health are released, burning the nose, eyes and respiratory organs.
Solder fat consists of paraffin, ammonium and zinc chloride, and deionized water. Solders well preheated areas that have undergone tinning procedure. After soldering aluminum parts, it is recommended to wash off the remaining flux, otherwise it will cause corrosion of the metal.
Liquid flux
Liquid flux is applied to the soldering area in a thin layer. When working with a soldering iron, it evaporates quickly, releasing scalding fumes. Flux F-64 contains fluorides, tetraethylammonium, corrosion inhibitors and ionized water. It destroys the oxide film well and helps to solder large aluminum workpieces. Used for soldering copper, aluminum, galvanized iron and other metals.
F-61 consists of triethanolamine, ammonium fluoroborate and zinc fluoroborate. Used for tinning and soldering aluminum alloys at temperatures up to 250°C. Castolin Alutin 51 L consists of cadmium, lead and 32% tin. Works most effectively at temperatures above 160°C.
Features of the substance
Active flux for soldering aluminum has significant differences from similar substances used to join products made of steel, brass and copper, just as the properties of these metals themselves differ. The flux composition contains substances that can easily dissolve the oxide film on the surface of aluminum. For soldering aluminum products, fluxes of various numbers are used.
The simplest are fluxes No. 8 and 9, however, their activity is not too great compared to those that include fluorine compounds. The choice is made based on the characteristics of a particular job. There are fluxes that are used for welding joints of parts without prior preparation. The most common type of flux is F-64.
The composition of the flux for aluminum soldering, number F-64, contributes to its increased activity, which makes it possible to successfully clean even uncleaned surfaces from the oxide film. The solution is colorless or light yellow.
Solder for soldering aluminum
Solder for soldering aluminum is made from zinc or aluminum. Additives are added to it to achieve various characteristics: to lower the melting point and increase strength. They are produced in America, Germany, France, Russia. Let's look at some of them.
A common and widely advertised solder for aluminum is HTS 2000. It is produced by a company from the USA. Practice shows its fragility: soldered parts allow air and moisture to pass through. It cannot be used without flux.
Castolin 192FBK based on zinc (97%) and aluminum (2%) is produced in France. The Castolin company produces solders 1827 and AluFlam-190, intended for soldering copper and aluminum at 280°C.
Castolin 192FBK is a tubular solder containing flux in the core. It is produced in the form of rods, 100 g of which costs 100-150 rubles. Solders small holes and cracks well.
Chemet Aluminum 13 is a solder used when welding parts at 640°C and above. It is based on aluminum (87%) and silicon (13%). The melting point of solder is about 600°C. It is produced in the form of rods, of which there are 25 pieces per 100 g. 100 g cost 500 rubles. The variety called Chemet Aluminum 13-UF has a hollow structure and contains flux in the core. Its cost for 12 rods, which weigh 100 g, is 700 rubles.
Aluminum solder is also produced at domestic enterprises. For soldering using a gas torch, grade 34A is used. It melts at a temperature of 525°C and solders AMts, AM3M, AMg2 alloys well. 100 g cost 700 rubles.
Grade A consists of 60% zinc, 36% tin and 2% copper. Melts at 425°C. Produced in rods weighing 145 g. The cost of one rod is 400 rubles.
SUPER A+ is produced in Novosibirsk and is an analogue of HTS-2000. Used together with SUPER FA flux. Costs 800 rubles. per 100 g. In the molten state it becomes viscous, you have to use steel tools to level it.
Other methods for joining copper and aluminum
For connecting dissimilar wires, in this case, copper and aluminum, there are other methods that are fully justified, as confirmed by many years of practice.
Crimping method
When laying and installing electrical wiring, it becomes necessary to permanently connect copper and aluminum wires by crimping using sleeves Source samelectrik.ru
The soldering method for connecting aluminum and copper wires is not always suitable, and the reasons may be different. Firstly, you simply may not have flux, and the connection needs to be made urgently. Secondly, it may not be possible to connect to ≈220 V, and thirdly, there may be no free space to reach with a soldering iron. For example, in an electrical distribution box (dose), all twists must be well insulated, but the usual rag tape is not suitable in this case, since it allows oxygen to pass through, which will contribute to the oxidation of aluminum and, as a result, burnout of the twist. Therefore, one of the most optimal insulation options in such situations will be a sleeve - a fragment of the required length, cut from a heat-shrinkable tube.
Heat shrink is applied to the twist so that it captures the insulation on one and the other wire at least a centimeter at a time. First, the sleeve is put on one of the wires, then a tight twist is made, the thermal strip is moved so that it is in the center, but at the same time captures the insulation on both sides. After this, all that remains is to heat up the heat-shrinkable tube, and it will crimp both the insulation on both sides and the twist itself. It is usually heated with an ordinary burning match, and the sleeve cools down in 1-2 minutes. After this, the access of oxygen to the connection is stopped.
Terminal blocks
One of the most common ways to connect wires is with terminal blocks Source elektroznatok.ru
Terminal blocks or, as electricians call them, terminal blocks, are used to connect homogeneous and dissimilar conductive metal cores. For joining in a plastic block, bolted or clamp terminals are used, which ensure 100% contact and the complete absence of the influence of aluminum oxidation on copper. Between the terminals there is a shunt plate made of a neutral metal (usually tinned copper or brass), which is not affected by the oxide film. The most important thing in the terminal block is a good clamping of both wires, which guarantees a long service life. The only contraindication for such a connection is high air humidity. If this happens in such a room, then it is better to use heat shrink.
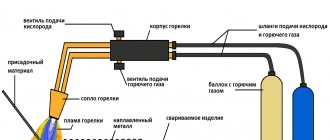
Aluminum soldering torches
You need to know how to solder with a gas torch. If the area of the parts is large and there is not enough soldering iron power, use a torch. It is better to use a gas one, since soldering aluminum with a gas torch has great capabilities. The burner quickly heats the joint of the parts almost to the melting temperature of aluminum. Flux with solder is applied to the joint, leveled with a soldering iron tip and hardened. The joint must be washed to remove any remaining soldering acid or other flux.
When working with burners, you must follow fire safety rules. There should be no flammable liquids or materials nearby.
Soldering techniques
Soldering fat
The main technological methods used in this process are the following:
- Degreasing the working surface with a clean rag soaked in solvent or acetone;
- Initial cleaning with a wire brush or coarse sandpaper;
- Applying flux to a cleaned work surface;
- Heating the metal with a torch;
- Solder melting under the jet of a burner flame to form a seam;
- Cooling and hardening of the seam;
- Removing scale and loose oxide from the resulting seam using a metal brush or sandpaper.
On a note. To control the heating temperature of the working surface, place a piece of solder on it. If it begins to melt and turn into a small droplet in the form of a ball, then it means that the metal has warmed up, and it is necessary to immediately begin soldering it.
How to choose the right flux
When you start choosing a flux, there are a couple of points to consider. First you need to understand what you are working with, because there may be other metal in the welding.
Then, the composition of the flux, it must contain active components, for example: Li (lithium), K (potassium), ZnCl2 (zinc chloride).
If you think about specific companies, then F64 is a standard flux. It contains elements with high activity, there will be no problems.
If you work with high temperatures, then 34A may be suitable for you, and if with low temperatures, then F61, it is sometimes called F61A.
If you solder aluminum and copper, then you can use Castolin 192 CW; its high-quality work has been proven by many positive reviews.
Fluxes – mixtures of salts
Many substances are used in fluxes - about 3 dozen - all of them are salts. Most of them are chlorides and fluorides. Additions of these salts to fluxes increase their specific properties: fluidity, wettability, and chemical activity.
The most famous of them are sodium chloride (table salt) and potassium chloride. Their melting point in pure form is 801 and 770 °C, respectively. Their density in the solid state is 2.165 and 1.984 g/cm3, and in the liquid state – 1.55 and 1.53 g/cm3.