Mohs hardness scale
The Mohs scale (mineralogical hardness scale) is a qualitative ordinal scale that characterizes the scratch resistance of various minerals.
Used to determine the relative hardness of mineral samples. Based on the ability of a harder material to scratch a softer material.
The scale contains 10 minerals as reference minerals, ranking them in order of increasing hardness from very soft (talc) to very hard (diamond).
All of the minerals in the table, except diamond, are relatively common and easy or inexpensive to obtain.
If a mineral scratches a standard, then its hardness is higher; if it is scratched by a standard, it means lower.
The Mohs scale was created in 1812 and named after its inventor, German geologist and mineralogist Friedrich Mohs. Since then, many different methods for determining hardness have been invented: the Brinell, Knoop, Rockwell, Shore, Vickers method.
Mohs hardness is a relative integer comparison of scratch resistance.
Other hardness measurement methods rely on indentation resistance. For testing, an “Indenter” is used, which is pressed into the test sample with a carefully measured force. The size or depth of the notch in the specimen and the magnitude of the force are then used to calculate the hardness value. Because each of these tests uses different apparatus and different calculations, they cannot be directly compared to each other.
The Mohs scale has become widespread because... The hardness test method is easy to perform, inexpensive and quickly understood by people.
Despite its lack of accuracy, the scale is useful for field geologists who use it for rough identification of minerals when examining easily identifiable samples or when more complex tests are not available.
Some use readily available items for a quick test. For example, a geologist may have a pocket knife that can be used to determine whether a sample is harder or softer than a Mohs value of 5-6.5.
Below is an extended table of substances, minerals, and precious stones:
| Substance or mineral | Mohs hardness |
| Pyrophyllite, molybdenite | 1-2 |
| Bauxite, coal | 1-3 |
| Limonite | 1-5 |
| Ice, sugar, gallium, strontium, indium, tin, barium, thallium, lead, graphite | 1,5 |
| Gypsum, calcium | 1,5-2 |
| Sulfur | 1,5-2,5 |
| Sylvite, glauconite, cadmium, selenium | 2 |
| Rock salt, cinnabar, chlorite, bismuth, amber | 2-2,5 |
| Muscovite | 2-3 |
| Silver, gold, galena, copper, biotite, mica | 2,5-3 |
| Aluminum, limestone, calcite, boric acid, nitrophoska | 3 |
| Aragonite, witherite, anhydrite | 3-3,5 |
| Pearl, brass, arsenic | 3-4 |
| Serpentine | 3-5 |
| Sphalerite, rhodochrosite, malachite, dolomite, cuprite, chalcopyrite, azurite, barite | 3,5-4 |
| Siderite, pyrrhotite, dolomite | 3,5-4,5 |
| Fluorite, phosphor bronze | 4 |
| Marble | 4-5 |
| Tooth enamel, asbestos, apatite, manganese, zirconium, palladium, obsidian | 5 |
| Titanite, monazite | 5-5,5 |
| Jade, uraninite, ilmenite, enstatite, porcelain stoneware (polished) | 5-6 |
| Magnetite | 5-6,5 |
| Nepheline, augite, arsenopyrite, actinolite, bustamite, cobaltite | 5,5-6 |
| Rhodonite, diopside, opal, red iron ore | 5,5-6,5 |
| Titanium, germanium, niobium, rhodium, uranium | 6 |
| Rutile, pyrite, prehnite, plagioclase, orthoclase, amazonite, andesine, anorthoclase, benitoite, helvite, iridium | 6-6,5 |
| Silicon | 6,5 |
| Jasper | 6,5-7 |
| Agate, zoisite, epidote, cassiterite, pyrolusite | 6-7 |
| Marcasite | 6-7,5 |
| Granite, tanzanite, spodumene, olivine, jadeite, axinite, chrysoprase, jadeite | 6,5-7 |
| Sillimanite, garnet | 6,5-7,5 |
| Quartz, stone pebbles, amethyst, aventurine, forsterite, osmium, silicone, rhenium, vanadium | 7 |
| Tourmaline, cordierite, almandine, boracite, cordierite, danburite | 7-7,5 |
| Zircon, andalusite, euclase, hambergite, sapphirine | 7,5 |
| Emerald, hardened steel, tungsten, spinel, beryl, beryllium, aquamarine, red beryl, ganite, painite | 7,5-8 |
| Topaz, cubic zirconia | 8 |
| Chrysoberyl, alexandrite, choltite | 8,5 |
| Porcelain tiles (unpolished) | 8,5 |
| Corundum, ruby, sapphire, alundum, chrome | 9 |
| Moissanite, boron | 9,5 |
| Carborundum | 9-10 |
| Diamond, carbonado | 10 |
© 2014-2019 All rights to materials on the site are protected in accordance with the legislation of the Russian Federation.
NOT ONE BLOOD
Oddly enough, the most accessible material for protecting dials has nothing to do with glass - at least at the molecular level. Plastic, which is called organic glass, sometimes plexiglass, is actively used in various inexpensive mechanical and electronic watches. The material under the Plexiglas brand was created in 1928, and its industrial production began in 1933. The appearance of plexiglass in the period between the two world wars was not accidental, and the engine of its spread was the development of aviation, which required a combination of strength, optical transparency, shatterproofness, that is, safety for the pilot, resistance to moisture and technical fluids.
Organic glass consists entirely of thermoplastic resin, and the production process is represented by two main methods: extrusion (extrusion of a hot polymer mass through a gap of a certain width and thickness) and casting (when a hot polymer mass is poured between two layers of glass or metal, and the size of the gap between the sheets is determined thickness of the future sheet).
Plastic glass is easy to scratch, but not so easy to break
The main advantage of plastic for the watch industry is its ability to easily take any shape, obediently following the will of designers. The relative softness is also reflected during use: plexiglass is easy to scratch, but not so easy to break. Plexiglas is easy to manufacture and maintain: polishing or replacing such glass is easy and inexpensive. It is a pity that it will have to be changed or polished often, especially if the owner of the watch leads an active lifestyle.
Mohs hardness scale for assessing the hardness of metals.
Here is a list of hardness coefficients for some metals that everyone is likely to encounter in their daily life, especially when coming into contact with jewelry:
- Tin: 1.5
- Zinc: 2.5
- Gold: 2.5-3
- Silver: 2.5-3
- Aluminum: 2.5-3
- Copper: 3
- Copper: 3
- Bronze: 3
- Nickel: 4
- Platinum: 4-4.5
- Steel: 4-4.5
- Iron: 4.5
- Palladium: 4.75
- Rhodium: 6
- Titan: 6
- Reinforced steel: 7-8
- Tungsten: 7.5
- Tungsten carbide: 8.5-9
PHYSICAL PROPERTIES
| Mineral color | iron black |
| Stroke color | grey |
| Transparency | opaque |
| Shine | metal |
| Cleavage | imperfect in |
| Hardness (Mohs scale) | 4,5 |
| Kink | hackly |
| Strength | malleable |
| Density (measured) | 7.3 – 7.87 g/cm3 |
| Radioactivity (GRapi) | |
| Magnetism | ferromagnet |
Why is it important to know the hardness of metals?
When German geologist Friedrich Mohs created the scale we use today, he applied a simple principle to determine the hardness of any material: what materials can scratch it, and what materials it can scratch.
For example, platinum, which has a hardness of 4-4.5, can be scratched by all materials that have a higher Mohs scale. For example, topaz, which has a coefficient of 8, can alternately scratch any material that has a lower coefficient (for example, gold, the hardness of which is rated at 2.5-3 points).
From the table above you can see which metals can scratch others and which ones can scratch them. This is valuable information as it can tell you which precious metals can be stored together and which cannot.
Also, this information about the hardness of metals will help determine which products made from precious alloys are more reliable to wear.
APPLICATION
Iron is one of the most used metals, accounting for up to 95% of global metallurgical production. Iron is the main component of steels and cast irons - the most important structural materials. Iron can be part of alloys based on other metals - for example, nickel. Magnetic iron oxide (magnetite) is an important material in the production of long-term computer memory devices: hard drives, floppy disks, etc. Ultrafine magnetite powder is used in many black and white laser printers mixed with polymer granules as a toner. This uses both the black color of the magnetite and its ability to stick to the magnetized transfer roller. The unique ferromagnetic properties of a number of iron-based alloys contribute to their widespread use in electrical engineering for magnetic cores of transformers and electric motors. Iron(III) chloride (ferric chloride) is used in amateur radio practice for etching printed circuit boards. Ferrous sulfate heptate (ferrous sulfate) mixed with copper sulfate is used to combat harmful fungi in gardening and construction. Iron is used as an anode in iron-nickel batteries and iron-air batteries. Aqueous solutions of ferrous and ferric chlorides, as well as its sulfates, are used as coagulants in the purification processes of natural and waste water in the water treatment of industrial enterprises.
| Molecular weight | 55.85 g/mol |
| origin of name | Possibly Anglo-Saxon origin |
| IMA status | valid, first described before 1959 (before IMA) |
How to apply the hardness scale for metals.
When you decide to buy a product made of a precious metal, but are undecided about which material to choose, the Mohs hardness scale will help.
By comparing the coefficients, you will make a preliminary choice and be able to decide whether this product is also suitable for you in terms of price.
For example, platinum is much more durable than silver, and in general, harder ones last longer with constant wear. However, platinum is also much more expensive than silver, so you need to consider whether you are willing to pay the extra price for durability.
PROPERTIES
In its pure form under normal conditions it is a solid. It has a silver-gray color and a pronounced metallic luster. The mechanical properties of iron include its level of hardness on the Mohs scale. It is equal to four (average). Iron has good electrical and thermal conductivity. The last feature can be felt by touching an iron object in a cold room. Because this material conducts heat quickly, it removes most of it from your skin in a short period of time, which is why you feel cold. If you touch, for example, wood, you will notice that its thermal conductivity is much lower. The physical properties of iron include its melting and boiling points. The first is 1539 degrees Celsius, the second is 2860 degrees Celsius. We can conclude that the characteristic properties of iron are good ductility and fusibility. But that's not all. Also, the physical properties of iron include its ferromagnetism. What it is? Iron, whose magnetic properties we can observe in practical examples every day, is the only metal that has such a unique distinctive feature. This is explained by the fact that this material is capable of magnetization under the influence of a magnetic field. And after the end of the action of the latter, the iron, the magnetic properties of which have just been formed, remains a magnet for a long time. This phenomenon can be explained by the fact that in the structure of this metal there are many free electrons that are able to move.
Hardness of metal alloys.
The Mohs scale for each metal indicates the hardness in its pure state, i.e. without any other materials mixed with it.
However, in reality, almost all metals used in jewelry are combined with others to create a stronger or cheaper material.
For example, gold is often mixed with nickel, zinc, copper and other metals to give it extra hardness.
Likewise, when carbon is added to tungsten, which has a hardness rating of 7.5 in its pure form, the resulting tungsten carbide will have a hardness rating of 8.5-9 on the Mohs Hardness Scale.
Mineral hardness test kit
ORIGIN
Origin telluric (terrestrial) iron is rarely found in basalt lavas (Uifak, Disko Island, off the western coast of Greenland, near Kassel, Germany). At both locations, pyrrhotite (Fe1-xS) and cohenite (Fe3C) are associated with it, which is explained by both the reduction by carbon (including from the host rocks) and the decomposition of carbonyl complexes such as Fe(CO)n. In microscopic grains, it has more than once been established in altered (serpentinized) ultrabasic rocks, also in paragenesis with pyrrhotite, sometimes with magnetite, due to which it arises during reduction reactions. Very rarely found in the oxidation zone of ore deposits, during the formation of swamp ores. Findings have been recorded in sedimentary rocks associated with the reduction of iron compounds with hydrogen and hydrocarbons. Almost pure iron was found in lunar soil, which is associated with both meteorite falls and magmatic processes. Finally, two classes of meteorites - stony-iron and iron - contain natural iron alloys as a rock-forming component.
The Hardest. Mohs scale. Chemistry - Simple
How to determine the hardness of a material? Very simple! To do this, you need to use a hardness scale.
The video describes what the Mohs hardness scale is and how to use it. Enjoy watching!
Possible duplicates found
17 minutes is too much)))
The Mohs hardness of a material is the resistance its surface exhibits when someone tries to scratch it with another material. Hardness depends on the degree of cohesion of the intra-atomic structure of the material.
The Mohs scale is a conventional scale of reference minerals for assessing the hardness of materials by scratching. The scale itself is presented below.
Mohs hardness scale
Hardness of minerals according to the Mohs scale
The Mohs scale (mineralogical hardness scale) is a set of reference minerals for determining relative hardness by scratching. 10 minerals, arranged in order of increasing hardness, were taken as standards.
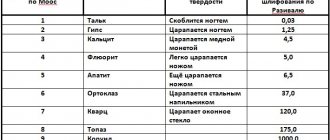
Consists of 10 hardness standards: talc - 1; gypsum - 2; calcite - 3; fluorite - 4; apatite - 5; orthoclase - 6; quartz - 7; topaz - 8; corundum - 9; diamond - 10.
Minerals with an index below 7 are considered soft, while those above 7 are considered hard.
In general, the bulk of natural compounds have a hardness of 2 to 6.
The hardness scale was proposed in 1811 by the German mineralogist Friedrich Mohs.
The hardness of a stone is the resistance that its surface offers when you try to scratch it with another stone or other object; hardness is a measure of the coherence of the atomic structure of a substance. The hardness of the same stone can be different in different directions. Kyanite stands out among other minerals due to the large difference in hardness in different directions: its hardness varies from 5 to 7, and in some directions the sample can be scratched with a knife, but not in others.
STRUCTURE
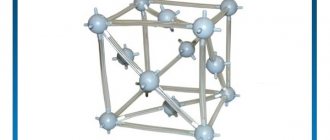
Two modifications of the iron crystal lattice
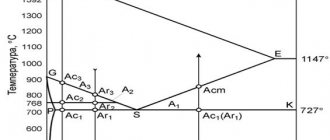
Several polymorphic modifications have been established for iron, of which the high-temperature modification - γ-Fe (above 906°) forms a lattice of a face-centered cube of the Cu type (a0 = 3.63), and the low-temperature modification - the α-Fe lattice of a centered cube of the α-Fe type (a0 = 2.86). Depending on the heating temperature, iron can be found in three modifications, characterized by different crystal lattice structures:
- In the temperature range from the lowest to 910 ° C - a-ferrite (alpha ferrite), which has a crystal lattice structure in the form of a centered cube;
- In the temperature range from 910 to 1390°C - austenite, the crystal lattice of which has the structure of a face-centered cube;
- In the temperature range from 1390 to 1535 ° C (melting point) - d-ferrite (delta ferrite). The crystal lattice of d-ferrite is the same as that of a-ferrite. The only difference between them is the different (larger for d-ferrite) distances between the atoms.
When liquid iron is cooled, primary crystals (crystallization centers) appear simultaneously at many points in the cooled volume. With subsequent cooling, new crystalline cells are built around each center until the entire supply of liquid metal is exhausted. The result is a granular structure of the metal. Each grain has a crystal lattice with a certain direction of its axes. With subsequent cooling of solid iron, during the transitions of d-ferrite to austenite and austenite to a-ferrite, new crystallization centers may appear with a corresponding change in grain size
Household hardness measuring instruments
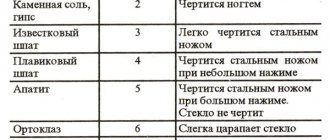
Sometimes, to determine hardness, you have to use the means that are at hand, although in some cases they are not accurate enough (pencil - 1, table salt - 2, fingernail - 2.5, copper coin - 3, iron nail - 4, glass - 5, steel knife – 6, file – 7). When determining hardness, a fresh mineral surface should always be tested.
For practical purposes it is good to remember:
- the nail leaves a scratch on plaster and softer substances;
- ordinary window glass is slightly softer than feldspar;
- The steel blade of a knife is slightly harder than feldspar, approaching quartz in hardness, and scratches glass easily.
RESERVES AND PRODUCTION
Iron is one of the most common elements in the solar system, especially on the terrestrial planets, in particular on Earth. A significant part of the iron of the terrestrial planets is located in the cores of the planets, where its content is estimated to be about 90%. The iron content in the earth's crust is 5%, and in the mantle about 12%.
Iron is quite widespread in the earth's crust - it accounts for about 4.1% of the mass of the earth's crust (4th place among all elements, 2nd among metals). In the mantle and earth's crust, iron is concentrated mainly in silicates, while its content is significant in basic and ultrabasic rocks, and low in acidic and intermediate rocks. A large number of ores and minerals containing iron are known. Of greatest practical importance are red iron ore (hematite, Fe2O3; contains up to 70% Fe), magnetic iron ore (magnetite, FeFe2O4, Fe3O4; contains 72.4% Fe), brown iron ore or limonite (goethite and hydrogoethite, respectively FeOOH and FeOOH nH2O ). Goethite and hydrogoethite are most often found in weathering crusts, forming so-called “iron hats”, the thickness of which reaches several hundred meters. They can also be of sedimentary origin, falling out of colloidal solutions in lakes or coastal areas of the seas. In this case, oolitic, or legume, iron ores are formed. Vivianite Fe3(PO4)2·8H2O is often found in them, forming black elongated crystals and radial aggregates. The iron content in sea water is 1·10−5-1·10−8%. In industry, iron is obtained from iron ore, mainly from hematite (Fe2O3) and magnetite (FeO·Fe2O3). There are various ways to extract iron from ores. The most common is the domain process. The first stage of production is the reduction of iron with carbon in a blast furnace at a temperature of 2000 °C. In a blast furnace, carbon in the form of coke, iron ore in the form of agglomerate or pellets, and flux (such as limestone) are fed from above, and are met by a stream of forced hot air from below. In addition to the blast furnace process, the process of direct iron production is common. In this case, pre-crushed ore is mixed with special clay, forming pellets. The pellets are fired and treated in a shaft furnace with hot methane conversion products, which contain hydrogen. Hydrogen easily reduces iron without contaminating the iron with impurities such as sulfur and phosphorus, which are common impurities in coal. Iron is obtained in solid form and is subsequently melted in electric furnaces. Chemically pure iron is obtained by electrolysis of solutions of its salts.
Linear hardness
Linear hardness is determined by the absolute hardness scale, not the Mohs scale. Here is the absolute hardness scale:
Talc – 1 – Can be scratched with a fingernail Gypsum – 3 – Scratched with a fingernail Calcite – 9 – Scratched with a copper coin Fluorite – 21 – Easily scratched with a knife Apatite – 48 – Hardly scratched with a knife Orthoclase – 72 – Scratched with a file Quartz – 100 – Scratches window glass Topaz – 200 – Easily scratches quartz Corundum – 400 – Easily scratches topaz Diamond – 1600 – Not scratched by anything (and at the same time easily scratches corundum)