Steel hardening
Hardening is a heat treatment operation of metal. It consists of heating the metal to a critical temperature at which the crystal lattice of the material changes , or to a temperature at which the phase dissolves in the matrix, which exists at a low temperature.
It is important to understand:
- After reaching a critical temperature, the metal undergoes rapid cooling.
- After hardening, the steel acquires the structure of martensite (named after Adolph Martens) and therefore becomes hard.
- Hardening increases the strength of steel. The metal becomes even harder and more wear-resistant.
- A distinction should be made between conventional quenching of the material and quenching to obtain excess vacancies.
Hardening modes differ in the speed of the process and heating temperature. There are also differences in the duration of exposure at a given temperature and cooling rate.
Temperature selection for hardening
The decision at what temperature to harden the metal is determined by the chemical composition of the steel.
There are two types of hardening:
- full;
- incomplete.
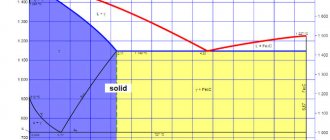
Based on the diagram of critical points, one can see that hypoeutectoid steel during the process of complete hardening should be heated above the Ac3 point by 30–50 degrees C. As a result, the steel will have a homogeneous austenite structure. Subsequently, under the influence of the cooling process, it will turn into martensite.
Figure No. 1. Critical points .
Incomplete hardening is more often used for tool steel. The purpose of incomplete hardening is to reach a temperature at which the process of formation of excess phases takes place. Heating of steel occurs in the temperature range from Ac1 - Ac2 . At the same time, some amount of ferrite remaining after hardening of the steel will remain in the martensite structure.
To harden hypereutectoid steel, it is better to maintain a temperature 20–30 degrees higher than Ac1 - incomplete hardening. Because of this, cementite will be retained during heating and cooling, which increases the hardness of martensite. When hardening, hypereutectoid steel should not be heated above the specified temperature. This may affect hardness.
Hardening temperature
The main criteria on the basis of which hardening modes are divided into types are the heating temperature and the speed of the technical process. There are also differences in such parameters as:
- time interval of exposure at certain temperature indicators;
- speed of the cooling procedure.
In general, based on the “heating temperature,” hardening can be of two types. Let's look at them briefly.
Complete hardening
Hypoeutectoid steel is processed by complete hardening. It is heated so that the final temperature exceeds the critical point Ac3 by 30°-50°. Then the mixture of ferrite and cementite is completely transformed into austenite. With further cooling, a predominantly martensitic structure is formed.
Hardening is incomplete
Tool steels are most often subjected to the incomplete hardening procedure. Carrying out heat treatment of this type has the goal of heating the product until the process of formation of excess phases begins. In this case, the following temperature range must be observed:
Ac1≤Т≤ Ac2, where
- T – heating temperature;
- Ac1, Ac2 – critical points. In the first (+727°C), recrystallization begins - pearlite is transformed into austenite. In the second (+768°C) α-Fe transforms into β-Fe and the steel loses its magnetic properties.
If this temperature range is maintained, the martensite structure will retain a certain amount of ferrite after quenching the steel.
Incomplete hardening of a hypereutectoid alloy is of the highest quality if the product is heated to a level exceeding Ac1 by 20°C-30°C. Then, during the process of heating and cooling, cementite will not be transformed. Because of this, the hardness of martensite will increase. If the heating temperature of the part goes beyond the above range, this characteristic may, on the contrary, worsen.
Cooling rate
The martensite structure is obtained by rapid cooling of austenite at the moment when the temperature of the steel contributes to the least stability of austenite (about 650-550 degrees).
When moving to the temperature zone in which martensitic transformation occurs (below 240 degrees), slow cooling is applied. As a result, the resulting structural stresses have time to level out while the hardness of the resulting martensite does not decrease.
To carry out successful heat treatment, it is very important to choose the right hardening medium. Often the following can be used as a quenching medium:
- water;
- sodium hydroxide solution (5–10%) or table salt;
- mineral oil.
To harden carbon steel, it is better to use water at a temperature of 18 degrees. Oil is suitable for hardening alloy steel.
Cooling Features
As is known, austenite is least stable at temperatures 550°С≤Т≤650°С. And the structure of martensite is formed when conditions are created for accelerated cooling of the alloy until its temperature index enters precisely this range. When the temperature falls below +240°C, the martensitic transformation is ensured by slow cooling. This technological solution leads to the fact that the stresses that arise in the metal body have time to level out. Moreover, without reducing the hardness of the formed martensite.
Successful heat treatment requires the correct choice of hardening medium. As such, the most commonly used are:
- mineral quenching oil;
- an aqueous solution of table salt (NaCl + H2O) or sodium hydroxide (NaOH);
- actually, water.
It is better to harden steel with alloying additives using oil. It is recommended to carry out this procedure with carbon alloys by cooling with water.
Characteristics of steel: hardenability and hardenability
The important characteristics of steel - hardenability and hardenability - should not be confused.
Hardenability
This characteristic indicates the ability of steel to gain hardness after hardening. There are types of steel that are difficult to harden and after the heat treatment process the steel becomes insufficiently hard. They say about such material that it “did not accept hardening.”
The hardness of martensite is related to the degree of distortion of its crystal lattice. A lower carbon content in martensite contributes to less distortion in the crystal lattice, which means the hardness of the steel will be lower. If the steel contains less than 0.3% carbon, then the hardenability of such an alloy is low, and usually such alloys are not hardened.
Hardenability
This characteristic can indicate how deeply the steel has been hardened. When hardening, the surface of a steel part cools faster than the core . This occurs because the surface is in direct contact with the cooling fluid, which removes heat. And the central part of the steel part gives off its heat through the thickness of the metal and the surface, where it is absorbed by the coolant.
Hardenability is affected by the critical hardening rate - the lower it (speed), the deeper the steel is hardened. For example, coarse-grained steel, which has a low critical hardening rate, is annealed deeper than fine-grained steel, which has a high critical hardening rate.
The depth of hardenability depends on the initial structure of the alloy being hardened, the heating temperature and the quenching medium. The hardenability of steel is determined by fracture, microstructure and hardness.
Characteristics of steel
In the context of this topic, steel has two important characteristics.
Hardenability
This characteristic reflects the fact how capable the steel is of becoming hard after undergoing the hardening procedure. There are alloys whose properties practically do not change as a result of this heat treatment, that is, the hardness remains at an insufficient level. They say about such a metal: “does not accept hardening.”
Metallurgy explains the high hardness of carbon-containing martensite by the distortion of its crystalline cells. This factor makes plastic deformation of the material difficult. The hardness index increases with increasing amount of carbon. In numbers it looks like this: the value of this parameter, established using the Rockwell method with the content of the element carbon (C) in steel at the level:
- 0.1%, equal to 30HRC;
- 0.7% is 64HRC.
But a further increase in the amount of carbon in the alloy does not lead to a significant increase in the hardness index. All this is displayed on the graph.
The following designations are used on it:
- pos. “1” – the heating temperature exceeds the Ac3 point;
- pos. “2” – the heating temperature of the product is 770°C, which is higher than only point Ac1;
- pos. “3” is an indicator of martensite hardness.
Typically, alloys with a carbon content of less than 0.3% are not subjected to the hardening procedure due to their low degree of hardening.
Hardenability
This characteristic indicates the depth of hardening of the steel. During this process, the core of the part cools more slowly than its surface. This phenomenon is explained by direct contact of the outer layer with a cooling substance that absorbs thermal energy. The situation is different with the central fragment of the product. Its heat is transferred through the thickness of the metal to the surface area, and there it is absorbed by the same cooling substance.
Hardenability is a characteristic derived from the critical hardening rate. This is understood as the lowest rate of supercooling of all austenite to the martensitic structural transformation. The depth of hardening is inversely proportional to this parameter. That is, the lower the speed of the above process, the deeper the hardening of the metal occurs. This is clearly manifested in alloys with large and small grains. The former are calcined to a greater depth than the latter, since they have a low critical speed.
Types of steel hardening
There are many ways to harden metal. Their choice is determined by the composition of the steel, the nature of the product, the required hardness and cooling conditions. Stepwise, isothermal and light hardening are often used.
Hardening in one environment
Referring to the graph of cooling curves for various hardening methods, you can see that hardening in one environment corresponds to curve 1. It is easy to perform such hardening. However, it is not suitable for every steel part. Due to the rapid decrease in temperature in steel of variable cross-section, temperature unevenness and high internal stress occur in the temperature range. This can cause the steel part to warp and crack.
Figure No. 2. Cooling curves .
A high carbon content in steel parts can cause volumetric changes in structural stresses, and this, in turn, threatens the appearance of cracks.
Hypereutectoid steels, which have a simple shape, are best quenched in one environment. To harden more complex shapes, hardening in two environments or step hardening is used.
Hardening in two environments (in Figure No. 2 this is curve 2) is used for tools made of high-carbon steel. The method itself consists in the fact that the steel is first cooled in water to 300-400 degrees , after which it is transferred to an oil environment, where it remains until it has completely cooled.
Step hardening
During stepwise hardening (curve 3), the steel part is first placed in a salt bath. The temperature of the bath itself must be higher than the temperature at which the martensitic transformation occurs (240–250 degrees) . After the salt bath, the steel is mixed into oil or air. Using step hardening, you don’t have to worry about the part warping or cracks forming in it.
The disadvantage of this hardening is that it can only be used for carbon steel workpieces with a small cross-section (8–10 mm). Step hardening can be used for parts made of alloy steel with a large cross-section (up to 30 mm).
Isothermal hardening
Isothermal hardening on the graph corresponds to curve 4. Hardening is carried out similarly to step hardening. However, steel is aged longer in a hot bath. This is done in such a way as to cause complete disintegration of the austenite . In the diagram, the shutter speed is shown on the S-shaped line by points a and b. Steel that has undergone isothermal hardening can be cooled at any speed. The cooling medium can be molten salts.
Advantages of isothermal hardening:
- steel is almost impossible to warp;
- no cracks appear;
- viscosity.
Light hardening
To carry out such hardening, a specially equipped furnace equipped with a protective environment is required. In production, to obtain a clean and bright surface of hardened steel, step hardening should be used. After this, the alloy is cooled in molten caustic alkali. Before the hardening process, the steel part is heated in a salt bath of sodium chloride at a temperature 30–50 degrees above point Ac1 (see “Critical point diagram”). The part is cooled in a bath at 180–200 degrees. The cooling medium is a mixture consisting of 75% potassium hydroxide, 25% sodium hydroxide, to which 6–8% water (based on the weight of salt) is added.
Hardening with self-tempering
Used in the production of tool steel. The main idea of hardening is to remove the steel part from the cooling medium until it is completely cooled. The withdrawal occurs at a certain point. A certain amount of heat is retained in the core of the steel part. Subsequent vacations are made at his expense .
After the steel product reaches the required temperature for tempering due to internal heat, the steel is placed in a quenching liquid for final cooling. Figure No. 3—Table of tarnish .
Tempering is controlled by the colors of tarnish (see Figure No. 3), which is formed on a smooth metal surface at 220–330 degrees.
Using self-tempering hardening, sledgehammers, chisels, plumbing hammers and other tools are made, which require high surface hardness while maintaining internal viscosity.
Types of hardening
Quite a lot of methods for hardening metal have been developed today. When choosing a specific one, you need to consider:
- chemical composition of the material;
- design features of the product;
- a given hardness indicator of the final product;
- cooling process conditions.
Quenching in one environment
To better understand the features of the hardening procedure, consider the image below. It shows graphs of cooling lines characteristic of various methods of such heat treatment.
The progress of hardening in one environment is shown by curve “1”. This method can be implemented without any particular difficulties. But it does not apply to all steel products. In particular, problems may arise with parts with variable cross-sections. An accelerated decrease in their temperature indicators leads to:
- formation of internal stresses;
- temperature unevenness.
The combination of these factors usually causes warping and cracking of such products.
When performing this hardening method, cracks can also form in parts made from alloys with a high content of the element carbon. In this case, volumetric transformations of structural stresses cannot be excluded. For hardening in one environment, products with a simple configuration made from hypereutectoid steels are better suited.
Hardening in two environments
This method is represented by curve “2” in the above figure. Hardening in two environments is most often applied to tools made from steels with a high carbon content. This heat treatment method is implemented in 2 stages:
- the product is first immersed in water, where its temperature will enter the range of 300°C≤T≤400°C;
- then the part is moved into an oil-cooling working environment. There the product remains until it cools completely.
Step hardening
The features of stepwise hardening are shown by curve “3”. This method is done like this:
- First, the steel product is placed in a bath of molten salts. Here it is necessary to control that the temperature of the coolant exceeds the martensitic transformation temperature (this range is 240°C≤T≤250°C);
- then the part is cooled in oil or in open space under natural environmental conditions.
With step hardening, the likelihood of warping or cracking of the product is zero. Workpieces with a cross-section of no more than 30 mm, made from steels with alloying additives, as well as products with a cross-section not exceeding 8 - a maximum of 10 millimeters, made of carbon alloys, are subject to such heat treatment.
Isothermal hardening
In the above figure, heat treatment of this type corresponds to curve number 4. The technique for performing it is similar to the previous method. The difference lies in the length of time the alloy is kept in a bath of molten salts. For isothermal hardening, this time interval is longer.
This technological solution ensures comprehensive decomposition of austenite. On the graph, the shutter speed is shown by points “a” and “b” on the S-shaped line. There are no restrictions on the cooling rate of the alloy subjected to isothermal hardening - it can take values from any range. This heat treatment method has another advantage: the metal of the final product acquires toughness.
Light hardening
Carrying out light hardening requires the use of a specially equipped furnace. It must contain a protective environment. To obtain a light surface for the workpiece, which also does not have visible flaws, it is recommended to use step hardening. Upon completion, the steel must be cooled in a molten substance with the following chemical formula: NaOH is a caustic alkali. Before the hardening procedure, the product is heated in equipment called a salt bath filled with sodium chloride. The temperature should exceed the Ac1 point by 20°C-30°C. During cooling, the temperature of the medium is maintained in the range of 180°C-200°C. It includes:
- caustic soda (NaOH) – 25%;
- caustic potassium (KOH) – 75%.
This mixture is diluted with water in an amount of about six to eight percent of the total mass of alkaline components.
Hardening with self-tempering
This method is used in the production of tool steel. The essence of the technology is the removal of a steel product from the cooling environment until it cools completely. After this operation, thermal energy is retained in the thickness of the metal. At her expense, in fact, further vacation is carried out.
But you need to perform subsequent actions related to this procedure while monitoring the temperature of the part. Only when this characteristic reaches the value required for tempering is the product moved to a quenching environment, where it is finally cooled.
Control of the tempering itself is carried out on the basis of tarnish colors. They represent a spectrum of different shades that appear on the surface of the alloy when an oxide film forms on it. This phenomenon occurs when the metal temperature varies in the range 220°С≤Т≤330°С.
Self-tempering hardening is used in the manufacture of hammers for masons and mechanics; chisels of all kinds, from scarpels to cross-mixers; sledgehammers, both sharp- and blunt-nosed. In general, for tools that require high surface hardness without sacrificing toughness.
Cooling methods during hardening
When steel products are rapidly cooled during hardening, there is a risk of large internal stresses occurring, which leads to warping of the material and sometimes cracks. To avoid this, where possible, it is better to cool steel parts in oil. Carbon steel, for which such cooling is not possible, is better cooled in water.
In addition to the cooling environment, the internal stress of steel products is affected by how they are immersed in the cooling environment. Namely:
- products with a thick and thin part in the quenching liquid with the bulky part first;
- If the product has an elongated shape (drills, taps), it must be immersed strictly vertically, otherwise they may warp.
Sometimes it is not necessary to harden the entire part, but only part of it. Then local hardening is applied. The product is not completely heated, but the entire part is immersed in the quenching liquid.
Cooling methods
When hardening steel parts, which is carried out with accelerated cooling, the likelihood of significant internal stresses occurring is very high. For this reason, metal warping occurs. Even cracking is possible. Preventing these negative phenomena is possible by cooling products in an oil environment, of course, if their production technology allows this.
A different approach is relevant for carbon steels. They cannot be cooled in oil. Therefore, this operation must be performed in water.
In addition to the cooling medium, the method of immersing workpieces into it is important from the point of view of the formation of internal stresses. In this case, the following rules should be followed:
- It is necessary to immerse parts, the design of which includes thin and thick fragments, into the hardening substance, starting with the larger one;
- drills, tools with which internal threads are cut - taps - in general, products characterized by an elongated configuration should be immersed without allowing their longitudinal axis to deviate from the vertical. Then they won't warp.
There are cases when it is necessary to harden only part of the part. This problem is solved by using local heat treatment. Only the required fragment of the product is heated, and the entire part is subject to immersion in the quenching liquid.
Defects during hardening of steel
- Insufficient hardness . Occurs if there was a low heating temperature, short holding time at operating temperature, or insufficient cooling rate. The fix is to apply a more energetic environment; anneal and then harden.
- Overheating . Occurs if a steel part is heated to a temperature exceeding the permissible one. When overheated, a coarse-grained structure is formed, which leads to brittleness of the part. Can be corrected: by annealing and hardening at the right temperature.
- Burnout . When a steel part is heated to a high temperature close to the melting point (1200–1300 degrees) in an oxidizing atmosphere. Oxygen penetrates into steel products and oxides form along the grain boundaries. This kind of steel cannot be repaired.
- Oxidation and decarbonization . In this case, scale (oxides) form on the surface of steel parts, and carbon burns out in the surface layers of the steel. This marriage cannot be fixed. To prevent defects, ovens with a protective atmosphere should be used.
- Warping and cracks . They arise due to internal stress. Cracks are an irreparable defect. Warping can be removed by straightening or straightening.