Machine parts and mechanisms are mainly made of steel. Particularly critical or loaded parts are made of carbon steels with mandatory hardening relative to the original state. The strength characteristics of materials are determined not only by their chemical composition, but also by the structure of the crystal lattice. Metals have different strength and hardness depending on the structure of the crystal lattice.
By heating and cooling metals, you can change the structure, and therefore influence their hardness and strength. The product at the workpiece level must be soft to facilitate machining. It becomes soft after annealing, when it has a pearlite crystal structure.
When steels are heated above the recrystallization temperature (GSE points on the iron-carbon diagram), the metal changes from α (alpha iron) to γ (gamma) iron, this crystal lattice structure is called austenite. If γ iron is quickly cooled, then the majority of the atoms will not have time to rearrange into their usual α lattice. This produces a solid product that has predominantly a martensite structure – i.e. solid solution of carbon in γ iron. The martensite lattice is significantly deformed and changes from cubic to tetragonal. A structure consisting of martensite will have the highest possible hardness.
In practice, finished parts have a martensite and pearlite structure in various proportions. The required ratio between structures, and therefore hardness and viscosity, is obtained using a subsequent heating operation called tempering. When tempered, some of the atoms from the γ lattice are rearranged into their usual α lattice, and internal stresses and, accordingly, hardness are reduced. Moreover, the higher the tempering temperature, the more atoms will rearrange, and the product will be less hard and more viscous.
Cooling modes during hardening
The most studied issues in materials science are the relationship between the chemical composition and the structure of the metal at certain temperatures. The most poorly studied area in hardening technology is methods, conditions and cooling modes. Meanwhile, it is in cooling that large reserves for controlling the structure and properties of the metal in finished products lie.
The main question of hardening is how intensely to cool? It would seem that if you cool it as quickly as possible, you will get maximum hardness, but at the same time, increased internal stresses will lead to the formation of cracks in the parts. The so-called hardening cracks, which are well known to all thermal experts. By cooling slowly, you will not achieve the required hardness and the part will need to be annealed and then undergo repeated heat treatment. Each brand has its own “critical” cooling rate, which ensures maximum hardness and will not lead to cracking. For example, 45X steel, depending on the type of coolant, can be hardened to HRC 45 or HRC 60. In order to “squeeze out” the maximum hardness, it is necessary to cool at a rate as close as possible to the critical speed, for a specific steel grade and workpiece geometry. From this we can draw a simple conclusion that the intensity of temperature reduction must be adjustable. There are only two widely used speeds: the cooling speed in water and in oil. Even taking into account that the intensity, in a small range, can be adjusted by temperature and circulation, the critical hardening rate may still not be obtained.
Water and oil media may only provide "critical" hardening rates in some applications. In addition, while working with water is relatively simple, oil hardening has specific features and disadvantages:
- insufficient cooling intensity for some brands;
- the ability to ignite, emit harmful vapors, smoke, coke on the walls of air ducts, etc.,
- good wetting of surfaces and, as a result, large removal of oil from workpieces, evaporation;
- changes in chemical composition under the influence of high temperatures;
- the need to wash the workpieces in detergents with further regeneration of oil films.
The disadvantages of traditional hardening options have contributed to the search for more optimal hardening mixtures and hardening methods, at least for some variants of workpieces and alloys. As a result, several options for hardening technologies and compositions have emerged that are better suited for certain types of products. The most widely used are liquid polymer concentrates combined with water. This technology first appeared in the Soviet Union in 1980.
Characteristics of water-polymer media
These compositions are a mixture of water and polymers in certain proportions
. Polymers are chemical compounds formed by long chains of macroparticles obtained by combining microparticles - monomers. This reaction is called polymerization. Mixing allows you to obtain a stable liquid with adjustable heat capacity, and therefore cooling capacity.
The basis of the composition of the liquid is water, even with altered properties. Therefore, there are restrictions on the use of water-polymer liquids. These environments are not recommended for hardening high-alloy tool and die steel grades, as well as parts of complex shapes or with variable cross-sections.
Polyacrylic iron salt is used as the initial polymer concentrate
brand PK-M. This polymer turned out to be cheap and had advantages over other polymers of similar composition. Polymer coolants were originally designed to replace oil to eliminate flammability. Soon they developed materials that were superior to oil in efficiency for some products. Other advantages of water-polymer media have also been discovered.
Average cooling results in various environments
| Characteristic | Oil I-20 | PC-M environment |
| Hardness | (HB ≤ 363) | 302 – 311 |
| Twist factor | (ext. 66-89) | 76 – 82 |
| Tensile strength (additional load 34-41 tf) | 34,6 – 36,0 | 35,4 – 37,4 |
| Tensile strength along the oblique washer (additional 34-42 tf) | 34,6 – 36,4 | 36,2 – 37,0 |
| Relative elongation (not less than 8.0) | 14 – 17 | 9,6 – 12,0 |
| Relative narrowing (not less than 40.0) | 53 – 59 | 50 – 53 |
| Impact strength (not less than 0.5 MPa) | 6,6 – 7,3 | 5,5 – 6,7 |
Process technology and methods of steel hardening
Everything about steel hardening technology: what it is, what it is needed for, what methods exist. Temperatures to which the metal is exposed. How the properties of steel change. Heating methods and cooling media. Heat treatment equipment. Defects during hardening.
Steel is hardened to increase its hardness, strength and wear resistance. This is one of the types of heat treatment in which the metal is first heated to temperatures that change its structural state, and then cooled so that it acquires the required physical and chemical composition and the necessary crystal structure. There are many ways to harden steel, leading to different results, but they all consist of two main cycles: heating to a critical point and cooling at a certain rate to a given temperature. Another technological operation used in the process of hardening metals is tempering, in which structural changes occur after heating to a low temperature with slow cooling. The ability to change the characteristics of steel through hardening is largely related to its original crystal structure and chemical composition, in which the most important components are carbon and alloying additives. They determine what the shape, size and configuration of the elements of the steel structure will be after its heat treatment.
What metals can be heated?
Metal hardening is a heat treatment that is most often applied to carbon and alloy steels in order to increase their hardness and improve strength characteristics. Heat treatment of non-ferrous metals, in particular tempering, annealing and hardening of copper, brass and bronze, as well as aluminum and titanium alloys, is somewhat less common. It should be noted that hardening of these compounds, unlike carbon steels, does not always lead to their strengthening; some copper alloys, on the contrary, then become more ductile and soft. Much more often, products made of non-ferrous metals are tempered to relieve stress after casting, stamping, rolling or drawing.
Equipment for heat treatment of steels
- muffle thermal furnaces;
- induction heating devices;
- installations for heating in melts;
- gas plasma installations;
- laser hardening devices.
The first three types can heat the entire volume of the product to the required temperature, while the latter can only heat the surface layer of the metal. In addition, furnaces for hardening metals are produced and widely used, in which heating is carried out in a vacuum or in an inert gas environment.
VIEW Induction heater on AliExpress from 7,506 rubles →
Quenching baths are represented by steel cooling tanks for various liquids, as well as special graphite crucibles and furnaces for molten salts or metals. Mineral oil, water and water-polymer mixtures are most often used as quenching liquids. Lead or tin are usually used for molten metals, and compounds of sodium, potassium and barium are used for molten salts. Quenching baths for liquid media have systems for heating and cooling the working fluid to the required temperature, as well as mixers for uniform distribution of the liquid and destruction of the steam jacket.
Classification of hot steel
Hardening in one environment
Step hardening
Step hardening takes place in two stages. In the first step, the product is placed in an environment with a temperature several tens of degrees higher than the point at which martensite begins to appear. After the temperature is equalized throughout the entire volume of the metal, the part is slowly cooled, as a result of which a martensitic structure is uniformly formed in it.
Isothermal hardening
During isothermal hardening, the product is also kept in a hardening bath at a temperature above the martensite point, but slightly longer. As a result, austenite is transformed into bainite, a type of troostite. This steel combines increased strength with ductility and toughness. In addition, after isothermal hardening, the residual stresses in the product are reduced.
Hardening with self-tempering
Light hardening
Light hardening is used for steel products whose surfaces should not be subject to oxidation during heat treatment. With this heat treatment, steel is heated in vacuum furnaces (see photo below) or in inert gas environments (nitrogen, argon, etc.), and cooled in non-oxidizing liquids or melts. This method is used to harden products that should not be subjected to further grinding, as well as parts that are critical to the carbon content in the surface layer.
Temperature for hardening
| steel grade | Temperature, C | ||
| hardening | annealing | vacations | |
| 15G | 800 | 780 | 200 |
| 65G | 815 | 790 | 400 |
| 15X, 20X | 800 | 870 | 400 |
| 30Х, 35Х | 850 | 880 | 450 |
| 40Х, 45Х | 840 | 860 | 400 |
| 50X | 830 | 830 | 400 |
| 50G2 | 805 | 830 | 200 |
| 40ХГ | 870 | 880 | 550 |
| OX13 | 1050 | 860 | 750 |
| 3Х13 | 1050 | 880 | 450 |
| 35ХГС | 870 | 860 | 500 |
| 30ХГСА | 900 | 860 | 210 |
| U7, U7A | 800 | 780 | 170 |
| P9, P12 | 1250 | 860 | 580 |
| R9F5, R9K5 | 1250 | 860 | 590 |
| R18F2 | 1300 | 900 | 590 |
| ШХ15 | 845 | 780 | 400 |
| 9ХС | 860 | 730 | 170 |
| R18K5F2 | 1280 | 860 | 580 |
| 1Х14Н18Б2БРГ | 1150 | 860 | 750 |
| 4Х14Н1482М | 1200 | 860 | 750 |
Determination of heating temperature in industrial production is carried out using contact and non-contact pyrometers. In recent decades, infrared devices have become widespread, making it possible to remotely measure the temperature at any point on the surface of a heated part. In addition, the approximate heating temperature of steel can be determined from color tables.
Properties of steel after hardening
During the heating process, carbon steel goes through a series of phase changes in its structure, during which its composition changes, as well as the shape and elements of the crystal lattice. At a critical temperature of 723 °C, the decomposition of cementite (iron carbide) begins in the still solid metal and the formation of a uniform solution of carbon in iron, called austenite. This state of carbon steel is the initial state for hardening.
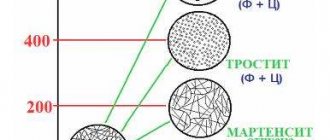
When cooled slowly, the austenite disintegrates and the metal returns to its original state. If the steel is cooled quickly, then the austenite does not have time to change, and at a certain cooling rate and threshold temperatures, crystal lattices and chemical compositions are formed, giving it different performance properties. This process is called hardening, and each type of it corresponds to a certain structure of already hardened steel, which has certain technical characteristics. The main phase states that are important during hardening are pearlite, sorbitol, troostite and martensite (see figure below).
The highest hardness is found in steel hardened to the state of martensite. In this way, the cutting tool is hardened, and the surfaces of parts exposed to friction during operation (bushings, cages, shafts, gears, etc.) are also hardened. After hardening with troostite, the steel becomes both hard and elastic. This type of heat treatment is applied to impact tools, as well as springs and spring shock absorbers. To obtain such properties of steel as wear resistance, elasticity and toughness, hardening to the sorbitol state is used. This heat treatment is used for rails and other structural elements operating under constant dynamic load. The listed phase states are characteristic of all carbon steels, but each grade is characterized by its own temperature ranges and cooling rates.
Cooling methods
- Cooling in one component. The product is immersed in liquid and remains in it until it cools completely.
- Intermittent hardening in two coolers. The product is first placed in a fast-cooling liquid, and after reaching a given temperature, it is transferred to a slow-cooling environment.
- Jet cooling. The heated part is intensively irrigated with a coolant flow (see photo below).
- Airflow The surface of the product is blown with a stream of air or inert gas.
In the practical application of hardening, all these types of cooling can have different variations or be combined with each other.
Cooling media
| № | Structure | Cooling medium | Hardness (HBW) |
| 1 | Martensite | Cold water | 500÷750 |
| 2 | Troostitis | Oil | 350÷500 |
| 3 | Sorbitol | Air | 250÷350 |
| 4 | Perlite | As the oven cools down | 150÷250 |
The influence of cooling speed on the final result
When hardening steel, cooling must occur at a rate that prevents the decomposition of austenite into ferrite and iron carbide, which begins to occur at temperatures below 650 °C. Further reduction in temperature should be carried out more slowly, since such a speed ensures a decrease in the internal stresses of the steel. Rapid and complete cooling in cold water produces martensite, which has maximum hardness but is quite brittle. With a rapid decrease in temperature by 200÷300 °C, the decomposition of austenite stops, and further slower cooling forms phase states in the steel with lower hardness, but with increased strength and wear resistance. The cooling rate is controlled by the type of quenching medium used and its temperature (see table below).
| № | Cooling medium | Cooling rate (deg/sec) |
| 1 | Air | 5 |
| 2 | Mineral oil | 150 |
| 3 | Water at room t° | 700 |
| 4 | Water at 80 °C | 1400 |
| 5 | 10% sodium chloride solution | 2100 |
| 6 | 10% sodium hydroxide solution | 1600 |